Introduction - Atoms and Molecules | 10th Science : Chapter 7 : Atoms and Molecules
Chapter: 10th Science : Chapter 7 : Atoms and Molecules
Atoms and Molecules
ATOMS AND MOLECULES
INTRODUCTION
You have learnt, in your lower classes that matter is around us everywhere. Matter is made of atoms. Curiously the idea of atom was first proposed by the Greek philosophers in the fifth century BC (BCE). But, their theory was more philosophical than scientific.
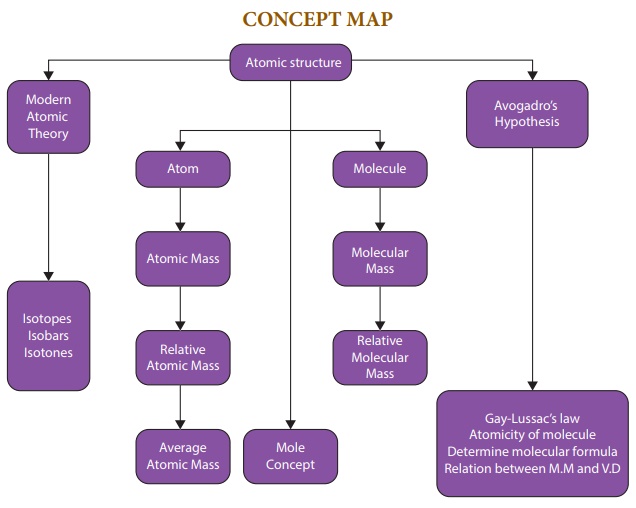
The first scientific theory of the atom was proposed by John Dalton. Few of the postulates of DaltonŌĆÖs theory about an atom were found incorrect by the later on studies made by J.J. Thomson, Rutherford, Neils Bohr and Schrodinger. In the light of the result of the researches most of the limitations of the DaltonŌĆÖs theory were removed and a new theory known as the modern atomic theory was put forward.
ŌĆśThe main postulates of modern atomic theoryŌĆÖ are as follows:
┬Ę An atom is no longer indivisible (after the discovery of the electron, proton, and neutron).
┬Ę Atoms of the same element may have different atomic mass. (discovery of isotopes 17Cl35, 17Cl37).
┬Ę Atoms of different elements may have same atomic masses (discovery of Isobars 18Ar40, 20Ca40).
┬Ę Atoms of one element can be transmuted into atoms of other elements. In other words, atom is no longer indestructible (discovery ofartificial transmutation).
┬Ę Atoms may not always combine in a simple whole number ratio (E.g. Glucose C6H12O6 C:H:O = 6:12:6 or 1:2:1 and Sucrose C12H22O11C:H:O = 12:22:11).
┬Ę Atom is the smallest particle that takes part in a chemical reaction.
┬Ę The mass of an atom can be converted into energy (E = mc2).
The modern atomic theory is the basis for all the studies of chemical and physical processes that involve atoms. You have studied the most fundamental ideas about an atom in your lower classes. Let us discuss some more concepts about atoms in this lesson.
Related Topics