Chapter: 10th Science : Chapter 7 : Atoms and Molecules
Atom and Atomic Mass
ATOM AND ATOMIC MASS
As you know, anything
that has mass and occupies space is called matter. Atoms are the building
blocks of matter. Since matter has mass, it must be due to its atoms. According
to the modern atomic theory, an atom contains subatomic particles such as
protons, neutrons and electrons. Protons and neutrons have considerable
mass, but electrons don't have such a considerable mass. Thus, the mass of
an atom is mainly contributed by its protons and neutrons and hence the
sum of the number of protons and neutrons of an atom is called its mass
number.
Individual atoms are
very small and it is difficult to measure their masses. You can measure the
mass of macroscopic materials in gram or kilogram. The mass of an atom is
measured in atomic mass unit (amu).
Atomic mass unit is
one-twelfth of the mass of a carbon- 12 atom; an isotope of carbon, which
contains 6 protons and 6 neutrons.
(Note: The symbol
ŌĆśamuŌĆÖ is no longer used in the modern system and instead, it uses the symbol
ŌĆśuŌĆÖ to denote unified atomic mass. The mass of a proton or neutron is
approximately 1 amu).
1. Relative Atomic Mass (RAM)
As an atom is very
small, its absolute mass cannot be determined directly. The early pioneers of
chemistry used to measure the atomic mass of an atom relative to an atom of
another element. They measured the masses of equal number of atoms of two or
more elements at a time, to determine their relative masses. They established
one element as a standard, gave it an arbitrary value of atomic mass and using
this value they measured the relative mass of other elements. The mass obtained
by this way is called relative atomic mass. In the beginning, the mass of
hydrogen atom was chosen as a standard and masses of other atoms were compared
with it, because of the existence of isotopic character of hydrogen (1H1,
1H2, 1H3). Later hydrogen atom was
replaced by oxygen atom as the standard. Now, the stable isotope of carbon
(C-12) with atomic mass 12 is used as the standard for measuring the relative
atomic mass of an element.
Relative atomic mass of
an element is the ratio between the average mass of its isotopes 1/12th
to part of the mass of a carbon-12 atom. It is denoted as Ar. It is
otherwise called ŌĆ£Standard Atomic WeightŌĆØ.
Relative Atomic Mass
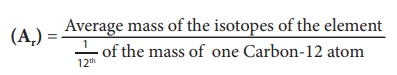
Modern methods of
determination of atomic mass by Mass Spectrometry uses C-12 as standard. For
most of the elements, the relative atomic mass is very closer to a whole number
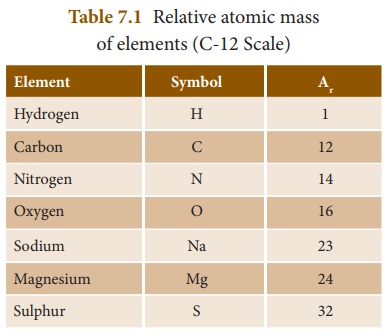
and it is rounded off to a whole number, to make calculations easier. Table 7.1
lists some of the elements of periodic table and their Ar values.
2. Average Atomic Mass (AAM)
How can one measure the
atomic mass of an element? It is somewhat more complicated because most of the
naturally occuring elements exist as a mixture of isotopes, each of which has its
own mass. Thus, it is essential to consider this isotopic mixture while
calculating the atomic mass of an element.
The average atomic mass
of an element is the weighted average of the masses of its naturally occurring
isotopes.
But, the abundance of
isotopes of each element may differ. So, the abundancy of all these isotopes
are taken into consideration while calculating the atomic mass. Then, what do
we mean by a weighted average? Let us consider an element which exists as a
mixture of 50% of an isotope having a mass of 9 amu, and 50% of another isotope
having a mass of 10 amu. Then, its average atomic mass is calculated by the
following equation:![]()
![]()
Average atomic mass = (Mass
of 1st isotope ├Ś % abundance of 1st isotope) + (Mass of 2nd isotope ├Ś %
abundance of 2nd isotope)
Thus, for the given
element the average
atomic mass = (9 ├Ś
50/100) + (10 x 50/100)
= 4.5 + 5 = 9.5 amu
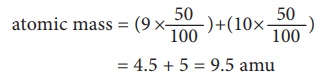
(Note: In the calculations involving percentages,
you need to convert percentage abundance into fractional abundance. For
example, 50 percent is converted into 50/100 or 0.50 as shown in the a foresaid
calculation.)
The atomic masses of
elements, given in the periodic table, are average atomic masses. Sometimes,
the term atomic weight is used to mean average atomic mass. It is observed,
from the periodic table that atomic masses of most of the elements are not
whole numbers. For instance, the atomic mass of carbon given in the periodic
table is 12.01 amu, not 12.00 amu. The reason is that while calculating the
atomic mass of carbon, both of its natural isotopes such as carbon-12. and
carbon- 13 are considered. The natural abundance of C-12 and C-13 are 98.90 %
and 1.10 % respectively. The average of the atomic mass of carbon is calculated
as follows:
Average atomic mass of
carbon
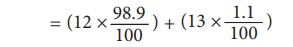
= (12 ├Ś 98.9/100 ) + (13 ├Ś
1.1/100)
= (12 ├Ś 0.989) + (13 ├Ś
0.011)
= 11.868 + 0.143 =
12.011 amu
So it is important to
understand that if it is mentioned that the atomic mass of carbon is 12 amu, it
refers to the average atomic mass of the carbon isotopes, not the mass of the
individual atoms of carbon.

Calculation of average atomic mass - Solved Examples
Example 1: Oxygen is the most
abundant element in both the EarthŌĆÖs crust and the human body. It exists
as a mixture of three stable isotopes in nature as shown in Table 7.3:
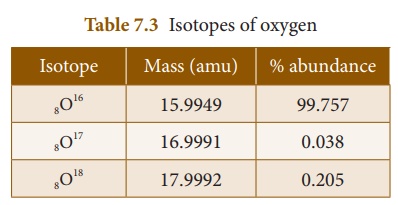
The atomic mass of oxygen = (15.9949
├Ś 0.99757) + (16.9991 ├Ś 0.00038) + (17.9992 ├Ś 0.00205)
= 15.999 amu.
Example 2: Boron naturally occurs
as a mixture of boron-10 (5 protons + 5 neutrons) and boron-11 (5
protons + 6 neutrons) isotopes. The percentage abundance of B-10 is 20 and that
of B-11 is 80. Then, the atomic mass of boron is calculated as follows:
Atomic mass of boron = (10
├Ś 20/100) + (11 ├Ś 80/100)
= (10 ├Ś 0.20) + (11 ├Ś
0.80)
= 2 + 8.8
= 10.8 amu
Related Topics