Chapter: 10th Science : Chapter 7 : Atoms and Molecules
Mole Concept
MOLE CONCEPT
So far we discussed
about matters in terms of individual atoms and molecules. Atomic mass units
provide a relative scale for the masses┬Ł of the elements. Since the atoms have
such small masses, no usable scale can be devised to weigh them in the
calibrated units of atomic mass units. In any real situation, we deal with ┬Łmacroscopic
samples containing enormous number of atoms┬Ł. Therefore, it is convenient to
have a special unit to describe a very large number of atoms. The idea of a
ŌĆśunitŌĆÖ to denote a ┬Łparticular number of objects is not new. For
example, the pair (2 items) and the dozen (12 items), are all
familiar units. Chemists measure atoms and molecules in ŌĆśmolesŌĆÖ. So, you
can now understand that ŌĆśmoleŌĆÖ denotes a number of particles.
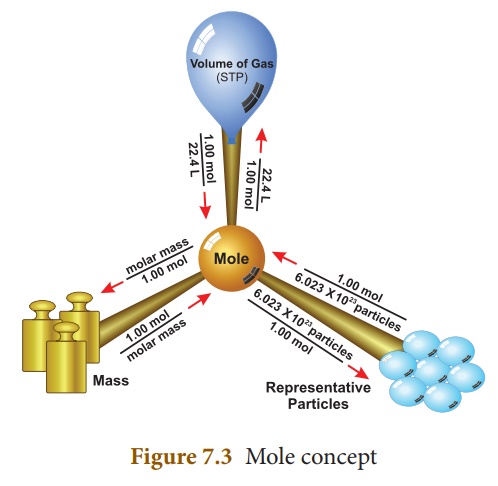
In the SI system, the mole (mol) is the amount of a substance that contains as many elementary entities (atoms, molecules, or other particles) as there are atoms in exactly 12 g (or 0.012 kg) of the carbon-12 isotope. The actual number of atoms in 12 g of carbon-12 is determined experimentally. This is called AvogadroŌĆÖs Number (NA), named after an Italian scientist Amedeo Avogadro who proposed its significance. Its value is 6.023 ├Ś 1023. So one mole of a substance contains 6.023 ├Ś 1023 entities. Thus, 5 moles of oxygen molecules contain 5 ├Ś 6.023 ├Ś1023 molecules.
Mole Concept: The study of the
collection of particles by using mole as the counting unit, in order to
express the mass and volume of such unit particles in a bulk of matter is known
as mole concept.
The number of moles of a
substance can be calculated by various means depending on the data available,
as follows:
┬Ę
Number of moles of molecules.
┬Ę
Number of moles of atoms.
┬Ę
Number of moles of a gas (Standard molar volume at STP = 22.4
litre).
┬Ę
Number of moles of ions.
Note:
STP-Standard Temperature
and Pressure(273.15 K,1.00 atm)
Mole of atoms:
One mole of an element
contains 6.023 ├Ś 1023 atoms and it is equal to its gram atomic mass.
i.e., one mole of oxygen
contains 6.023 ├Ś 1023 atoms of oxygen and its gram atomic mass is 16
g.
Mole of molecules:
One mole of matter
contains 6.023 ├Ś 1023 molecules and it is equal to its gram
molecular mass.
i.e., one mole of oxygen
contains 6.023 ├Ś 1023 molecules of oxygen and its gram molecular
mass is 32 g.![]()
![]()
Molar volume:
One mole of any gas
occupies 22.4 litre or 22400 ml at S.T.P. This volume is called as molar
volume.
Calculation of number of moles by Different modes
Number of moles
= Mass / Atomic Mass
= Mass / Molecular mass
= Number of Atoms / 6.023 ├Ś 1023
= Number of Molecules /
6.023 ├Ś 1023

Related Topics