Chapter: 10th Science : Chapter 7 : Atoms and Molecules
Relationship Between Vapour Density and Relative Molecular Mass
RELATIONSHIP BETWEEN
VAPOUR DENSITY AND RELATIVE MOLECULAR MASS
1. Relative molecular
mass: (Hydrogen scale)
The Relative Molecular
Mass of a gas or vapour is the ratio between the mass of one molecule of the
gas or vapour to mass of one atom of Hydrogen.
2. Vapour Density:
Vapour density is the
ratio of the mass of a certain volume of a gas or vapour, to the mass of an
equal volume of hydrogen, measured under the same conditions of temperature and
pressure.
Vapour Density (V.D.) =
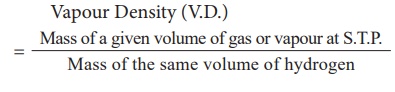
According to Avogadro's law, equal
volumes of all gases contain equal number of molecules.
Thus, let the number of
molecules in one volume = n, then
V.D. at S.T.P =

Cancelling 'n' which is
common, you get V.D.
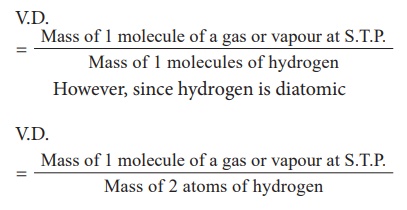
When you compare the
formula of vapour density with relative molecular mass, they can be represented
as V.D.
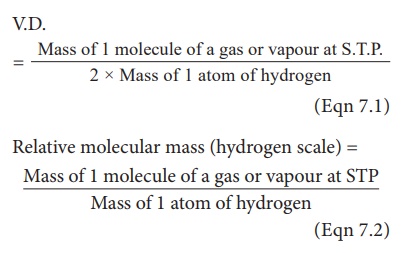
You can therefore
substitute the above equation to an Eqn 7.1 and arrive at the following formula
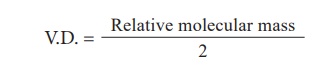
Now on cross
multiplication, you have
2 Ă— vapour density =
Relative molecular mass of a gas
(Or)
Relative molecular mass
= 2 Ă— Vapour density
Related Topics