Atoms and Molecules - Percent Composition | 10th Science : Chapter 7 : Atoms and Molecules
Chapter: 10th Science : Chapter 7 : Atoms and Molecules
Percent Composition
PERCENT COMPOSITION
So for, we were dealing
with the number of entities present in a given substance. But many times, the
information regarding the percentage of a particular element present in a
compound is required.
The percentage
composition of a Âcompound represents the mass of each element present in 100 g
of the compound.
Let us understand the
percentage Âcomposition of oxygen and hydrogen by taking the example of H2O.
It can be calculated using the formula
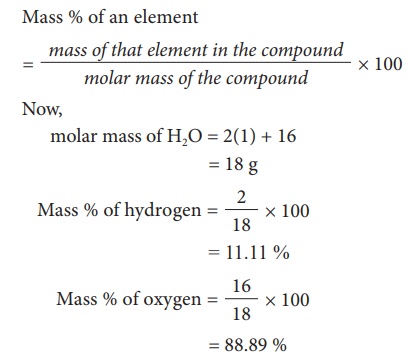
This percentage
composition is useful to determine the empirical formula and molecular formula.
Example 1: Find the mass percentage
Âcomposition of methane (CH4).
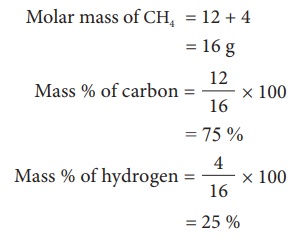
Related Topics