Chapter: Organic Chemistry: Structure and bonding
Atomic structure of carbon
ATOMIC STRUCTURE OF CARBON
Key Notes
Atomic orbitals
The
atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the
three p orbitals in the second shell.
The 1s and 2s orbitals are spherical in shape. The 2p orbitals are dumbbell in shape and can be assigned 2px, 2py or 2pz
depend-ing on the axis along which they are aligned.
Energy levels
The 1s orbital has a lower energy than the 2s orbital which has a lower energy than
the 2p orbitals. The 2p orbitals have equal energy (i.e. they
are degenerate).
Electronic configuration
Carbon
is in the second row of the periodic table and has six electrons which will
fill up lower energy atomic orbitals before entering higher energy orbitals
(aufbau principle). Each orbital is allowed a maximum of two elec-trons of
opposite spin (Pauli exclusion principle). When orbitals of equal energy are
available, electrons will occupy separate orbitals before pairing up (Hund’s
rule). Thus, the electronic configuration of a carbon atom is 1s2 2s2 2px1
2py1.
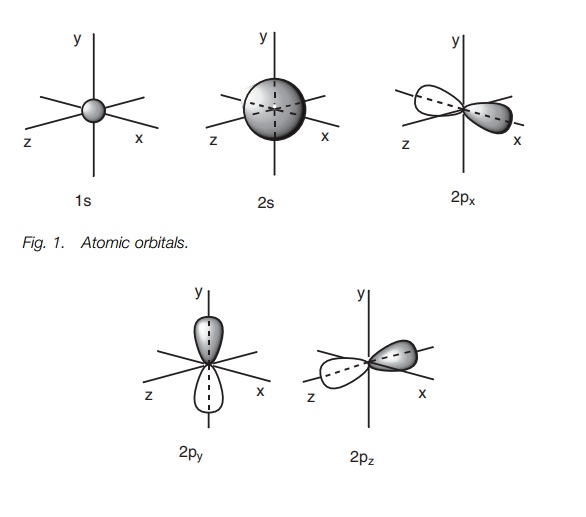
Atomic orbitals
Carbon has six electrons and is in row 2 of the
periodic table. This means that there are two shells of atomic orbitals
available for these electrons. The first shell closest to the nucleus has a
single s orbital – the 1s orbital. The second shell has a single
s orbital (the 2s orbital) and three p
orbitals (3x2p). Therefore, there are
a total of five atomic orbitals into which these six electrons can fit. The s orbitals are spherical in shape with
the 2s orbital being much larger then
the 1s orbital. The p orbitals are dumbbell-shaped and are
aligned along the x, y and z axes. Therefore, they are assigned the 2px, 2py
and 2pz atomic orbitals (Fig. 1).
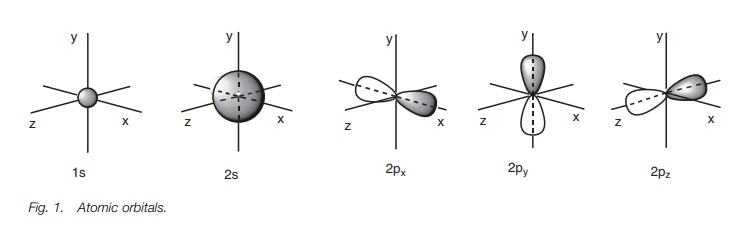
Energy levels
The atomic orbitals described above are not of
equal energy (Fig. 2). The 1s orbital has the lowest energy. The 2s orbital is next in energy and the 2p orbitals have the highest energies.
The three 2p orbitals have the same
energy, meaning that they are degenerate.
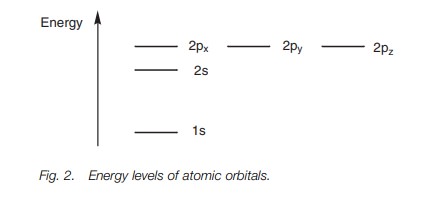
Electronic configuration
Carbon is in the second row of the periodic
table and has six electrons which will fill up the lower energy atomic orbitals
first. This is known as the aufbau
princi-ple. The 1sorbital is
filled up before the 2sorbital, which
is filled up before the 2p orbitals.
The Pauli exclusion principle states
that each orbital is allowed a maxi- mum of two electrons and that these
electrons must have opposite spins. There- fore, the first four electrons fill
up the 1s and 2s orbitals. The electrons in
each orbital have opposite spins and this is represented in Fig. 3 by drawing the arrows pointing up
or down. There are two electrons left to fit into the remaining 2p orbitals. These go into separate
orbitals such that there are two half-filled orbitals and one empty orbital.
Whenever there are orbitals of equal energy, electrons will only start to pair
up once all the degenerate orbitals are half filled. This is known as Hund’s rule.
The electronic configuration for carbon is 1s2 2s2 2px1
2py1. The
numbers in superscript refer to the numbers of electrons in each orbital. The
letters refer to the types of atomic orbital involved and the numbers in front
refer to which shell the orbital belongs.
Related Topics