Chapter: Organic Chemistry: Structure and bonding
Covalent bonding and hybridization
COVALENT BONDING AND HYBRIDIZATION
Key Notes
Covalent bonding
When two
hydrogen atoms approach each other, their 1s
atomic orbitals interact to form a bonding and an antibonding molecular orbital
(MO). A stable covalent bond is formed when the bonding MO is filled with a
pair of electrons and the antibonding MO is empty.
Sigma bonds
Sigma (σ) bonds are strong bonds with
a circular cross-section formed by the head-on overlap of two atomic orbitals.
Hybridization
The
electronic configuration of atomic carbon implies that carbon should form two
bonds. However, it is known that carbon forms four bonds. When carbon is part
of an organic structure, it can ‘mix’ the 2s
and 2p orbitals of the valence shell
in a process known as hybridization. There are three possible types of
hybridization – sp3, sp2 and sp hybridization.
Covalent bonding
A covalent bond binds two atoms together in a
molecular structure and is formed when atomic orbitals overlap to produce a molecular orbital – so called because
the orbital belongs to the molecule as a whole rather than to one specific
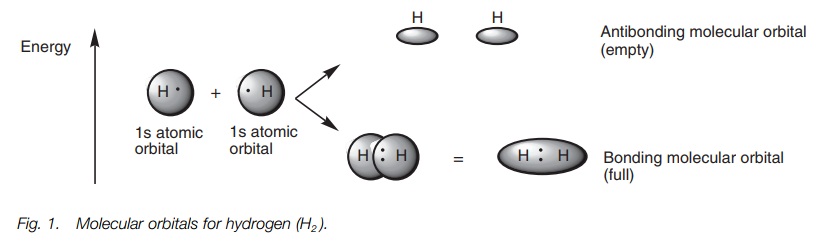
atom. A simple example is the formation of a hydrogen molecule (H2)
from two hydrogen atoms. Each hydrogen atom has a half-filled 1s atomic orbital and when the atoms
approach each other, the atomic orbitals interact to produce two MOs (the
number of resulting MOs must equal the number of original atomic orbitals, Fig. 1).
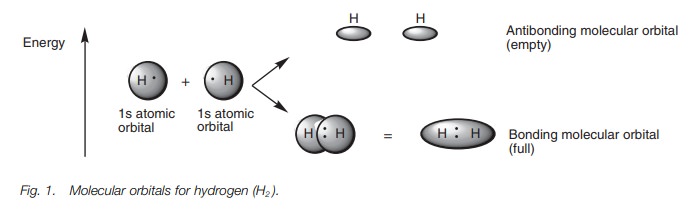
The MOs are of different energies. One is more
stable than the original atomic orbitals and is called the bonding MO. The other is less stable and is called the antibonding MO. The bonding MO is
shaped like a rugby ball and results from the combination of the 1s atomic orbitals. Since this is the
more stable MO, the valence electrons (one from each hydrogen) enter this
orbital and pair up. The antibonding MO is of higher energy and consists of two
deformed spheres. This remains empty. Since the electrons end up in a bonding
MO which is more stable than the original atomic orbitals, energy is released
and bond formation is favored. In the subsequent discussions, we shall
concentrate solely on the bond-ing MOs to describe bonding and molecular shape,
but it is important to realize that antibonding molecular orbitals also exist.
Sigma bonds
The bonding molecular orbital of hydrogen is an
example of a sigma (σ) bond: σ bonds have a circular cross-section and are formed by the head-on
overlap of two atomic orbitals. This is a strong interaction and so sigma bonds
are strong bonds. In future discussions, we shall see other examples of σ bonds formed by the interaction of atomic orbitals other than the
1s orbital.
Hybridization
Atoms can form bonds with each other by sharing
unpaired electrons such that each bond contains two electrons. We identified
that a carbon atom has two unpaired electrons and so we would expect carbon to
form two bonds. However, carbon forms four bonds! How does a carbon atom form
four bonds with only two unpaired electrons?
So far, we have described the electronic
configuration of an isolated carbon atom. However, when a carbon atom forms
bonds and is part of a molecular struc-ture, it can ‘mix’ the s and p orbitals of its second shell (the valence shell). This is known as
hybridization and it allows carbon
to form the four bonds which we observe in reality.
There are three ways in which this mixing
process can take place.
·
the 2s orbital is mixed
with all three 2p orbitals. This is
known as sp3
hybridization;
·
the 2s orbital is mixed
with two of the 2p orbitals. This is
known as sp2
hybridization;
·
the 2s orbital is mixed
with one of the 2p orbitals. This is
known as sp hybridization.
Related Topics