Chapter: Organic Chemistry: Structure and bonding
sp2 Hybridization
SP2 HYBRIDIZATION
Key Notes
Definition
In sp2 hybridization, a 2s orbital is ‘mixed’ with two of the 2p orbitals to form three hybridized sp2 orbitals of equal energy.
A single 2p orbital is left over
which has a slightly higher energy than the hybridized orbitals.
Electronic configuration
For
carbon, each sp2 hybridized
orbital contains a single unpaired electron. There is also a half-filled 2p
orbital. Therefore, four bonds are possible.
Geometry
Each sp2 orbital is shaped like a
deformed dumbbell with one lobe much larger than the other. The remaining 2p orbital is a symmetrical dumbbell. The
major lobes of the three sp2
hybridized orbitals point to the corners of a triangle, with the 2p orbital perpendicular to the plane.
Alkenes
Each sp2 hybridized carbon forms
three σ bonds
using three sp2 hybridized
orbitals. The remaining 2p orbital
overlaps ‘side on’ with a neighboring 2p
orbital to form a pi (π) bond. The π bond is weaker than the σ bond, but is strong enough
to prevent rotation of the C=C bond. Therefore, alkenes are planar, with each
carbon being trigonal planar.
Carbonyl groups
The
oxygen and carbon atoms are both sp2
hybridized. The carbon has three sp2hybridized
orbitals and can form threeσbonds, one of which is to theoxygen. The oxygen
has one sp2 orbital which
is used in the σ bond
with carbon. The p orbitals on carbon
and oxygen are used to form a π bond.
Aromatic rings
Aromatic
rings are made up of six sp2
hybridized carbons. Each carbon forms three σ bonds which results in a planar ring. The
remaining 2p orbital on each carbon
is perpendicular to the plane and can overlap with a neigh-boring 2p orbital on either side. This means
that a molecular orbital is formed round the whole ring such that the six π electrons are delocalized
around the ring. This results in increased stability such that aromatic rings
are less reactive than alkenes.
Aromatic rings
Conjugated
systems such as conjugated alkenes and α,β-unsaturated carbonyl compounds involve
alternating single and double bonds. In such systems, the p lobes of one π bond are able to overlap with the p lobes of a neighboring π bond, and thus give a small
level of double bond character to the connecting bond. This partial
delocalization gives increased stability to the conjugated system.
Definition
In sp2
hybridization, the s orbital is mixed
with two of the 2p orbitals (e.g. 2px and 2pz) to give three sp2
hybridized orbitals of equal energy. The remaining 2py orbital is unaffected. The energy of each hybridized
orbital is greater than the original s orbital but less than the original p orbitals. The remaining 2p orbital (in this case the 2py orbital) remains at its
original energy level (Fig. 1).
Electronic configuration
For carbon, there are four valence electrons to
fit into the three hybridized sp2
orbitals and the remaining 2p
orbital. The first three electrons are fitted into each of the hybridized
orbitals according to Hund’s rule such that they are all half- filled. This
leaves one electron still to place. There is
a choice between pairing it up in a half-filled sp2 orbital or placing it into the vacant 2py orbital. The usual prin-
ciple is to fill up orbitals of equal energy before moving to an orbital of
higher energy. However, if the energy difference between orbitals is small (as
here) it is easier for the electron to fit into the higher energy 2py orbital resulting in three half-filled sp2 orbitals and one
half-filled p orbital (Fig. 1).
Four bonds are possible.
Geometry
The 2py
orbital has the usual dumbbell shape. Each of the sp2 hybridized
orbitals has a deformed dumbbell shape similar to an sp3 hybridized
orbital. However, the difference between the sizes of the major and minor lobes
is larger for the sp2 hybridized
orbital.
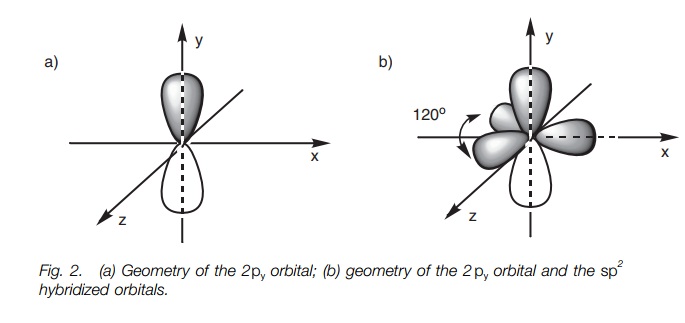
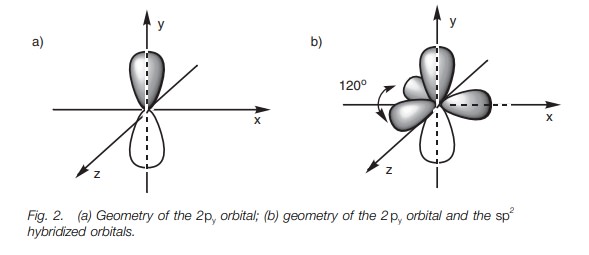
The hybridized orbitals and the 2py orbital occupy spaces as
far apart from each other as possible. The lobes of the 2py orbital occupy the space above and below the plane of
the x and z axes (Fig. 2a). The
three sp2 orbitals (major lobes shown only) will then
occupy the remaining space such that they are as far apart from the 2py orbital and from each
other as possible. As a result, they are all placed in the x–z plane pointing toward
the corner of a triangle (trigonal planar shape; Fig. 2b). The angle between each of these lobes is 120 . We are
now ready to look at the bonding of ansp2
hybridized carbon.
Alkenes
sp2 Hybridization
results in three half-filled sp2
hybridized orbitals which form atrigonal planar shape. The use of these
three orbitals in bonding explains the shape of an alkene, for example ethene
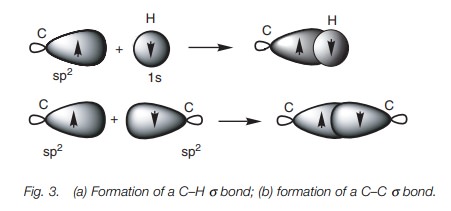
(H2C=CH2). As far as the C–H bonds are concerned, the hydrogen atom uses a
half-filled 1s orbital to form a
strong σ bond with a half filled sp2 orbital from carbon (Fig. 3a). A strong σ bond is also possible between the two carbon
atoms of ethene due to the overlap of sp2
hybridized orbitals from each carbon (Fig.
3b).
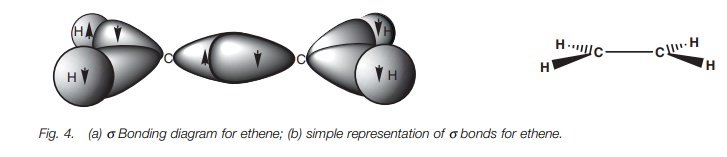
The full σ bonding diagram for ethene is shown in Fig. 4a and can be simplified as shown in Fig. 4b. Ethene is a flat, rigid molecule where each carbon is
trigonal pla-nar. We have seen how sp2
hybridization explains the trigonal planar carbons but we have not explained
why the molecule is rigid and planar. If the σ bonds were the only bonds present in ethene, the molecule would
not remain planar since rotation could occur round the C–C σ bond (Fig. 5).
Therefore, there must be fur-ther bonding which ‘locks’ the alkene into this
planar shape. This bond involves
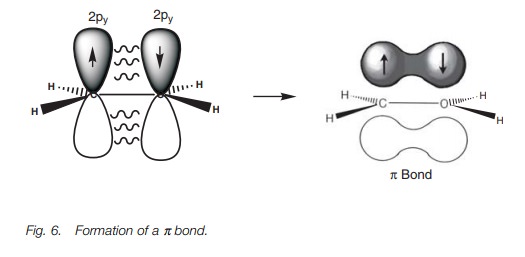
the remaining half-filled 2py orbitals on each carbon which overlap side-on to
pro-duce a pi (π) bond),
with one lobe above and one lobe below the plane of the mol-ecule (Fig. 6). This π bond prevents rotation round the C–C bond since the π bond would have to be broken to allow rotation. A π bond is weaker than aσ bond since the 2py orbitals overlap side-on, resulting in a weaker
overlap. The presence of a π bond also explains why alkenes are more
reactive than alkanes, since a π bond is more easily broken and is more likely
to take part in reactions.
Carbonyl groups
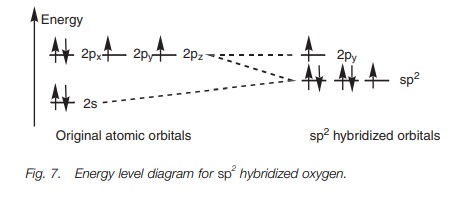
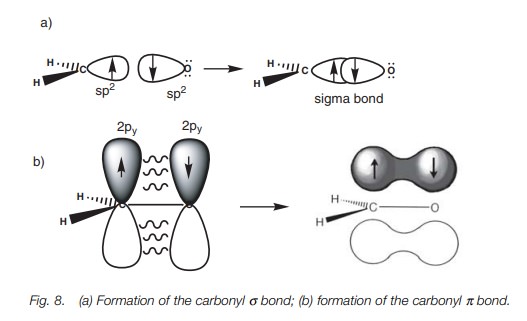
The same theory explains the bonding within a carbonyl group (C=O) where both the carbon and oxygen atoms are sp2 hybridized. The following energy level diagram (Fig. 7) shows how the valence electrons of oxygen are arranged after sp2 hybridization. Two of the sp2 hybridized orbitals are filled with lone pairs of electrons, which leaves two half-filled orbitals available for bonding. The sp2 orbital can be used to form a strong σ bond, while the 2py orbital can be used for the weaker π bond. Figure 8 shows how the σ and π bonds are formed in the carbonyl group and explains why carbonyl groups are planar with the carbon atom having a trigonal planar shape. It also explains the reactivity of carbonyl groups since the π bond is weaker than the σ bond and is more likely to be involved in reactions.
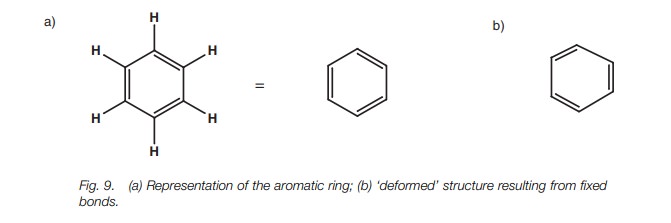
Aromatic rings
All the carbons in an aromatic ring aresp2hybridized which means
that eachcarbon can form three σ bonds and one π bond. In Fig. 9a, all
the single bonds are while each double bond consists of one σ bond and one π bond. However, this is an oversimplification
of the aromatic ring. For example, double bonds are shorter than single bonds
and if benzene had this exact structure, the ring would be deformed with longer
single bonds than double bonds (Fig. 9b).
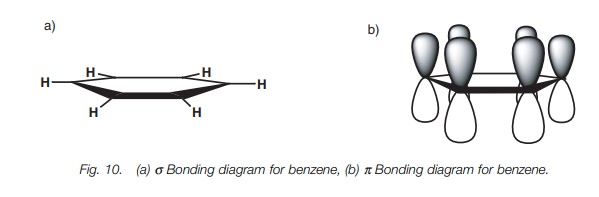
In fact, the C–C bonds in benzene are all the
same length. In order to understand this, we need to look more closely at the
bonding which takes place. Figure 10a
shows benzene with all its σ bonds and is drawn such that we are looking
into the plane of the benzene ring. Since all the carbons are sp2 hybridized, there is a 2py orbital left over on each
carbon which can overlap with a 2py
orbital on either side of it (Fig. 10b).
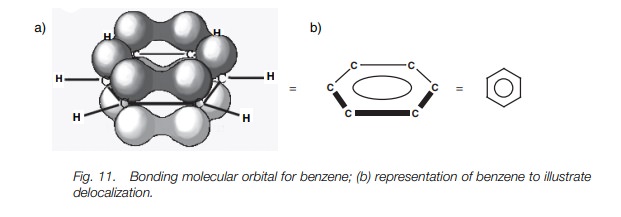
From this, it is clear that each 2py
orbital can overlap with its neigh-bors right round the ring. This leads to a
molecular orbital which involves all the 2py
orbitals where the upper and lower lobes merge to give two doughnut-like lobes
above and below the plane of the ring (Fig.
11a). The molecular orbital is symmetri-cal and the six π electrons are said to be delocalized around the aromatic ring
since they are not localized between any two particular carbon atoms. The
aromatic ring is often represented as shown in Fig. 11b to represent this delocalization of the π
Delocalization increases the
stability of aromatic rings such that they are less reactive than alkenes (i.e.
it requires more energy to disrupt the delocalized π system of an aromatic ring than it does to break the isolated π bond of an alkene).
Conjugated systems
Aromatic rings are not the only structures
where delocalization of π electrons can take place. Delocalization
occurs in conjugated systems where there are alternat- ing single and double
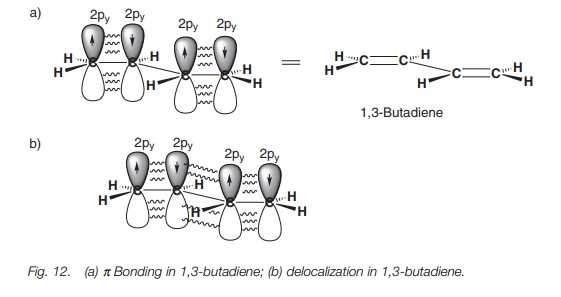
bonds (e.g. 1,3-butadiene). All four carbons in 1,3-butadiene are sp2 hybridized and so each of
these carbons has a half-filled p
orbital which can interact to give two π bonds (Fig. 12a).
However, a certain amount of overlap is also possible between the p orbitals of the middle two carbon
atoms and so the bond connecting the two alkenes has some double bond character
(Fig. 12b) – borne out by the
observation that this bond is shorter in length than a typical single bond.
This delocalization also results in increased stability. However, it is
important to realize that the conjugation in a conjugated alkene is not as
great as in the aromatic system. In the
latter system, the π
electrons are completely delocalized round the ring and all the bonds
are equal in length. In 1,3-butadiene, the π electrons are not fully delocalized and are more likely to be
found in the ter- minal C–C bonds. Although there is a certain amount of π character in the middle bond, the latter is more like a single
bond than a double bond.
Other examples of conjugated systems include α,β-unsaturated ketones and α,β- unsaturated esters (Fig. 13). These too have increased stability due to conjugation.
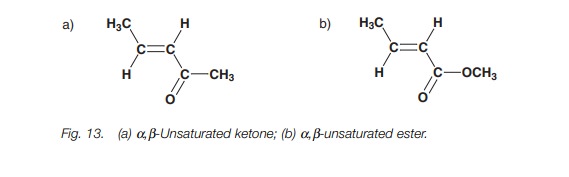
Related Topics