Chapter: Genetics and Molecular Biology: Chemotaxis
Assaying Chemotaxis
Assaying Chemotaxis
Sensitive and simple characterization of a cell’s
chemotactic ability is necessary for efficient study of the phenomenon. One
straightforward assay is the motility plates or swarm plates developed by
Adler. In one application, the medium in these plates contains the usual salts
neces-sary for cell growth, a low concentration of galactose, and a
concentra-tion of agar such that the medium has the consistency of vichyssoise.
Five hours after a drop of chemotactic cells is placed in the center, the plate
will contain a ring about two centimeters in diameter surrounding many of the
cells that remain where they were spotted. The ring expands outward with time
and ultimately reaches the edge of the plate. Usually, a second ring also forms
and moves behind the first.
The origin of the rings is straightforward.
Sensitive chemical tests show that the cells spotted in the center of the plate
gradually consume the galactose where they are placed (Fig. 22.1), but just
beyond the edge of the spot, galactose remains at its original concentration.
That is, the consumption of galactose creates a concentration gradient in
galactose at the edge of the spot. The bacteria on the edge of the spot detect
this
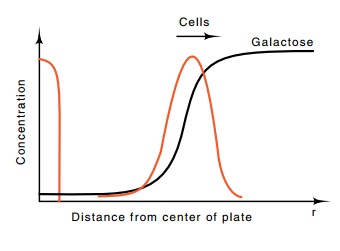
Figure
22.1 The concentra-tions of galactose
and cells as a function of distance r
from the center of the plate several hours after spotting cells.
gradient, swim toward higher galactose
concentrations, and, at the same time, consume the galactose in the new
position. Consequently, the cells move outward pursuing and consuming the
galactose. The second ring that forms on the galactose plates derives from
bacteria utilizing their endogenous energy sources to swim toward oxygen. If
tryptone medium is substituted for galactose salts medium, then multi-ple rings
form, the first ring from bacteria swimming toward the most active attractant,
the second toward a second attractant, and so on. In this case also, one of the
rings is formed by bacteria pursuing oxygen.
The capillary tube assay is more sensitive and
quantitative than the swarm plate assay. Instead of requiring the bacteria to
produce their own gradient of attractant concentration, the gradient is
produced by diffusion of the substrate. A short capillary tube is filled with
medium containing an attractant and is sealed at one end. The open end is
pushed into a drop of medium lacking attractant but containing cells, and the
tube is allowed to rest there an hour. As attractant diffuses out of the mouth
of the capillary, a gradient of attractant is produced in the region
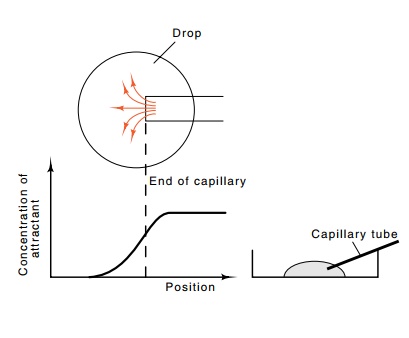
Figure
22.2 Diagram ofthe capillary tube
assay in which the capillary is in-serted in a drop of medium in a petri plate,
a top view showing diffusion out of a capillary to generate a gra-dient, and
the effective concentration as a function of position of the attractant after
several hours of diffusion.
(Fig. 22.2). The chemotactic bacteria in the drop
near the capillary end detect and swim up the gradient a short distance into
the capillary. At the end of the assay the capillary tube is removed, rinsed
from the outside, and the bacteria inside are blown out into a tube. These are
diluted and plated on petri plates. Since the number of bacteria having entered
the capillary can be accurately measured by counting the number of colonies on
the plates after incubation, the chemotactic ability of the cells can be
quantitated. Typically less than 100 nonmotile bacteria enter the capillary.
Several thousand motile but nonchemotac-tic bacteria normally enter a capillary
with or without attractant, and as many as 500,000 chemotactic bacteria will
enter a capillary tube that contains attractant.
Related Topics