Chapter: Essentials of Psychiatry: Antipsychotic Drugs
Antipsychotic Drugs: Adverse Effects
Adverse
Effects
Acute Extrapyramidal Side Effects (Dystonia, Parkinsonism, Akathisia)
Antipsychotic-induced
EPS occur both acutely and after chronic treatment. All antipsychotic
medications are capable of producing EPS. In general, first-generation
antipsychotics are more likely to cause EPS than second-generation
antipsychotics when the drugs are used at usual therapeutic doses. Among
second-generation drugs, clozapine and quetiapine have been shown to carry
mini-mal to no risk for EPS within the therapeutic dosage range. The
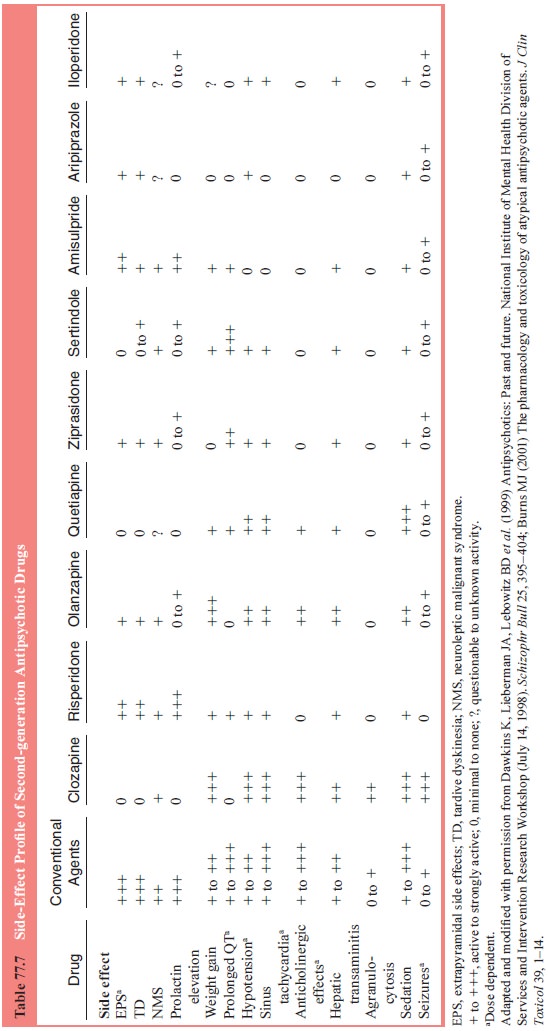
side-effect profiles for second-generation drugs and comparison with selected
other agents are summarized in Table 77.7.
Tardive Dyskinesia and Other Tardive Syndromes
TD is a
repetitive, involuntary, hyperkinetic movement disorder caused by sustained
exposure to antipsychotic medication. TD is characterized by choreiform
movements, tics and grimaces of the orofacial muscles, and dyskinesia of distal
limbs, often the paraspinal muscles, and occasionally the diaphragm (Glazer,
2000). Younger patients with TD tend to exhibit slower athetoid movements of
the trunk, extremities and neck (Marder, 1997). In addition to the more
frequently observed orofacial and chore-oathetoid signs of TD, tardive
dystonias (sustained abnormal postures or positions) and tardive akathisia
(persistent subjec-tive and/or objective signs of restlessness) have been
described (Casey, 1999). The abnormal movements of TD are usually in-creased
with emotional arousal and are absent when the individ-ual is asleep (Marder,
1997). According to the diagnostic criteria proposed by Schooler and Kane
(1982), the movements should be present for at least 4 weeks, and exposure to
antipsychotic drugs should have totaled at least 3 months. The onset of the abnormal
movements should occur either while the patient is receiving an antipsychotic
agent or within a few weeks of discontinuing the offending agent.
Prevalence
surveys indicate that mild forms of TD occur in approximately 20% of patients
who receive chronic treatment with conventional antipsychotic medication (Kane
and Lieber-man, 1992; Casey, 1995). Among the most significant predictors of TD
are older age, female gender, presence of EPS, diabetes mellitus, affective
disorders and certain parameters of neurolep-tic exposure such as dose and
duration of therapy (Casey, 1999).
For most
patients, TD dose not appear to be progressive or irreversible (Gardos et al., 1994). The onset of TD often
tends to be insidious with a fluctuating course (American Psychiatric
Association Task Force, 1992). With time, TD will either stabi-lize or improve
even if the antipsychotic medication is continued, although there are reports
of TD worsening during continued drug therapy (American Psychiatric Association
Task Force on TD, 1992; Gardos et al.,
1994). After discontinuation of antipsy-chotic medication, a significant
proportion of patients with TD will have remission of symptoms, especially if
the TD is of recent onset or the patient is young (Glazer et al., 1984). Unfortunately, withdrawal of antipsychotic agents is
seldom an option for pa-tients with serious psychosis (Marder, 1997).
The
American Psychiatric Association Task Force on TD (1992) issued a report in
which a number of recommendations were made for preventing and managing TD.
These include 1) establishing objective evidence that antipsychotic medications
are effective for an individual; 2) using the lowest effective dose of
antipsychotic drugs; 3) prescribing cautiously for children, elderly patients,
and patients with mood disorders; 4) examining patients on a regular basis for
evidence of TD; 5) considering alternatives to antipsychotic drugs, obtaining
informed consent and also considering a reduction in dosage when TD is
diag-nosed; and 6) considering a number of options if the TD worsens, including
discontinuing the antipsychotic medication, switching to a different drug, or
considering a trial of clozapine.
Although
a large number of agents have been studied for their therapeutic effects on TD,
there is no definitive drug treat-ment for it (American Psychiatric
Association, 2000). Second-generation antipsychotics, in particular clozapine,
have been used in clinical practice to treat TD, but there have been no
adequately controlled trials to date support this practice. Casey (1999),
how-ever, suggested that second-generation antipsychotics should be used as
first-line treatment for patients who have TD or are at risk for TD. Guidelines
for treating TD recommend using sec-ond-generation agents for mild TD symptoms,
and clozapine or a newer agent for more severe symptoms (McEvoy et al., 1999).
Neuroleptic Malignant Syndrome
Neuroleptic
malignant syndrome (NMS), another type of acute EPS, is characterized by the
triad of rigidity, hyperthermia (101– 104 F), and autonomic instability in
association with the use of an antipsychotic medication (American Psychiatric
Association, 1994). NMS is often associated with elevation of creatine kinase
(greater than 300 U/mL), leukocytosis (greater than 15 000 mm3) and change in
level of consciousness (American Psychiatric As-sociation, 2000). NMS can be of
sudden and unpredictable onset, usually occurring early in the course of
antipsychotic treatment, and can be fatal in 5 to 20% of untreated cases
(American Psychi-atric Association, 2000).
The
incidence of NMS varies from 0.02 to 3.23%, reflect-ing differences in criteria
(Caroff and Mann, 1993). Prevalence rates are unknown, but are estimated to
vary from 1 to 2% of pa-tients treated with antipsychotic medication (American
Psychi-atric Association, 2000). The relative risk of second-generation
antipsychotics for NMS is likely to be lower, but conclusive data is not yet
available (Burns, 2001). NMS has been reported with clozapine, risperidone,
olanzapine and quetiapine (Burns, 2001). Proposed risk factors include prior
episode of NMS, younger age, male gender, physical illness, dehydration, use of
high-potency antipsychotics, rapid dose titration, use of parenteral (IM)
prepa-rations and preexisting neurological disability (Caroff and Mann, 1993;
American Psychiatric Association, 2000).
If NMS is
suspected, the offending antipsychotic agent should be discontinued and
supportive and symptomatic treat-ment started (Marder 1997). Both dantrolene
and dopamine ago-nists such as bromocriptine have also been used in the
treatment of NMS (American Psychiatric Association, 2000). These agents,
however, have not shown greater efficacy compared with sup-portive treatment
(Caroff and Mann, 1993; Levenson, 1985).
The usual
course of treatment is between 5 and 10 days. Long-acting depot preparations
will prolong recovery time. After several weeks of recovery, treatment may be
cautiously resumed with a different antipsychotic medication with gradually
in-creased doses (American Psychiatric Association, 2000).
Metabolic Effects
Various
degrees of weight gain have been recognized as a com-mon problem with
conventional antipsychotic medications. Weight gain is an important issue in
the management of patients, because this adverse effect may be associated with
noncompli-ance and certain medical illnesses, such as diabetes mellitus,
cardiovascular disease, certain cancers and osteoarthritis (Lader, 1999;
Sussman, 2001; Kurzthaler and Fleischhacker, 2001).
Differences
have been discovered among second-generation antipsychotics with respect to
their ability to induce weight gain (Table 77.7). There is currently no
standard approach to the management of weight gain induced by antipsychotic
medication. Patient education prior to initiating treatment should be provided,
and regular exercise should be encouraged in all patients receiving
antipsychotic medication. Switching to other second-generation antipsychotics
with fewer propensities for producing weight gain may be the most efficient way
to deal with antipsychotic-induced weight gain.
Hematologic Side Effects
Antipsychotic
medications may cause blood dyscrasias, includ-ing neutropenia, leukopenia,
leukocytosis, thrombopenia and agranulocytosis. Leukopenia, usually transient,
commonly oc-curs early in treatment, and resolves spontaneously.
Chlorpro-mazine has been associated with benign leukopenia, which oc-curs in up
to 10% of patients (American Psychiatric Association, 2000; Kane and Lieberman,
1992). This phenomenon is even more common following clozapine administration
(Hummer et al., 1994).
Agranulocytosis
(granulocyte count less than 500/mm3) is a fatal side effect of antipsychotic
drugs. Approximately 0.32% of patients receiving chlorpromazine, and 1% treated
with clozapine will experience agranulocytosis (American Psychiatric
Associa-tion, 2000; Lieberman et al.,
1989). The risk of agranulocytosis is greatest early in treatment, usually
within the first 8 to 12 weeks of treatment (Novartis Pharmaceuticals, 2000).
It tends to occur slightly more often in women, the elderly and young patients
(less than 21 years old). Agranulocytosis from clozapine is usually re-versible
if the drug is withdrawn immediately (Lieberman et al., 1988). Olanzapine is not associated with severe
agranulocytosis (Beasley et al.,
1996a, 1996b; Dossenbach et al.,
2000). Despite these encouraging studies, there are a number of case studies
re-porting agranulocytosis during treatment with olanzapine (and quetiapine) in
patients who had suffered this adverse event dur-ing previous clozapine
exposure (Tolosa-Vilella et al.,
2002).
Before
initiating treatment with clozapine, patients in the USA must be registered in
a program that ensures that they receive weekly monitoring of their white blood
cell (WBC) count during the first 6 months of treatment (Marder, 1997).
Clozapine is prescribed on a weekly basis unless the WBC count is less than
3500/mm3 or if there is a substantial drop in the WBC count (Marder, 1997). If
the WBC count is 3000 to 3500/mm3 and the absolute neutrophil count (ANC) is
greater than 1500/mm3, patients should be monitored twice weekly. If the WBC
count is 2000 to 3000/mm3 or the ANC count is 1000 to 1500/mm3, clozapine
treatment should be discontinued and patients should be monitored daily. If the
WBC count falls be-low 2000/mm3 or the ANC is less than 1000/mm3, clozapine
should be discontinued and bone marrow aspiration should be considered
(American Psychiatric Association, 2000). If this occurs, the patient should be
given immediate intensive treat-ment and considered protective isolation.
Current guidelines require weekly monitoring for 1 month after the termination
of clozapine treatment (American Psychiatric Association, 2000). Guidelines on
the use of clozapine vary between dif-ferent countries.
Other Side Effects
Sedation
is the single most common side effect among low-potency conventional
antipsychotics, as well as clozapine, zoteapine and quetiapine (American
Psychiatric Association, 2000; Young et
al., 1998). Although sedation is often beneficial at the beginning of
treatment to calm down an anxious or ag-gressive patient, it usually impairs
functioning during long-term treatment (Hummer and Fleischhacker, 2000). Most
patients usually develop tolerance over time, or it may be possible to minimize
sedation by dose reduction or by shifting most of the medication to night to
reduce daytime sleepiness (Hummer and Fleischhacker, 2000).
Antipsychotic
medications can lower the seizure thresh-old to some degree (Kane and
Lieberman, 1992). Seizure is more common with low-potency first-generation
antipsychotics and clozapine (American Psychiatric Association, 2000).
Clozapine is associated with dose-related increase in seizures. For example,
Devinsky and colleagues (1991) reported that doses of clozapine below 300
mg/day have a seizure rate of about 1%, doses between 300 and 600 mg/day have a
seizure rate of 2.7%, and doses above 600 mg/day have a rate of 4.4%.
Strategies to reduce the risk for seizures include slower dose titration, a
lower dose and the addi-tion of an anticonvulsant agent (i.e., valproic acid)
(Hummer and Fleischhacker, 2000).
Related Topics