Chapter: Essentials of Psychiatry: Mood Stabilizers
Acute Mania
Acute
Mania
General Management Considerations
The goal
of treatment should be to suppress completely all symp-toms of mania and return
the patient to his or her mental status quo
ante. Mood, thinking and behavior should normalize. The patient should act and feel like herself or himself. For economic
or social reasons, it is sometimes necessary to discharge a patient before
total remission of symptoms has occurred. In such cases it is imperative that a
patient continues to take medication as pre-scribed and is protected from a
recrudescence of manic symp-toms or a rapid slide into acute depression.
Pharmacotherapy
The
mechanism of action of antimanic drugs is poorly understood. It is not as clear
what neural systems are involved in the mechanism of antimanic drugs. That few
significant changes in neurotransmit-ter levels have been measured suggests
that the site of action may be at the receptor, intracellular level, or
second-messenger systems.
Lithium
For many
patients with hypomania, lithium by itself can induce a total remission. For
patients with full-blown mania, however, an adjunctive antipsychotic or
antianxiety agent may be required to treat intolerable psychosis or excitement.
Lithium
is rapidly and completely absorbed after oral ad-ministration. It is not
protein bound and does not undergo me-tabolism. Peak plasma levels are achieved
within 1.5 to 2 hours for standard preparations or 4 to 4.5 hours for
slow-release forms. Lithium’s plasma half-life is 17 to 36 hours. Ninety-five
percent of the drug is excreted by the kidneys, with excretion proportion-ate
to plasma concentrations. Because lithium is filtered through the proximal
tubules, factors that decrease glomerular filtration rates will decrease
lithium clearance. Sodium also is filtered through the proximal tubules, so a
decrease in plasma sodium can increase lithium reabsorption and lead to
increased plasma lithium levels. Conversely, an increase in plasma lithium
levels can cause an increase in sodium excretion, depleting plasma so-dium.
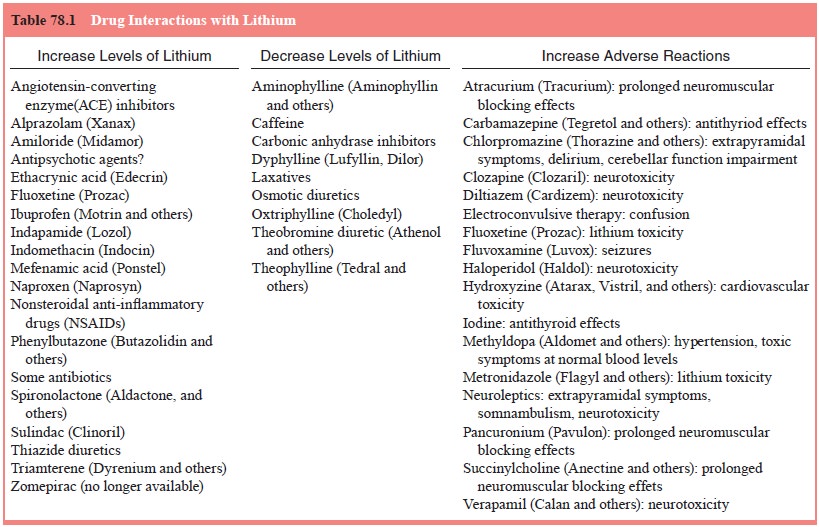
Many other drugs affect lithium levels (Table 78.1).
Tests
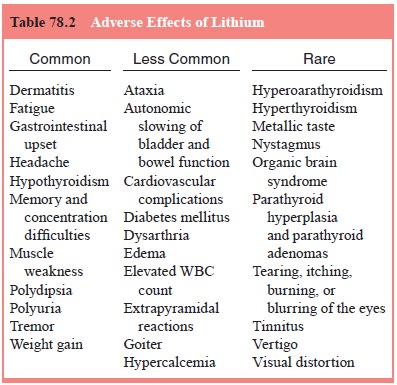
that should be done before lithium is started include a complete blood count,
electrocardiography, electrolyte determi-nations, and renal and thyroid panels
(Table 78.2). Lithium dos-age may be based on a plasma concentration sampled 12
hours after the last dose, or the drug may be gradually titrated to a dose that
is tolerated and within the range usually considered “ther-apeutic”. As with
any drug, approximately five half-lives must elapse for steady state to be
achieved. For an average adult, this takes about 5 days for lithium (longer in
the elderly or in patients with impaired renal function). To treat acute mania,
plasma concentrations should typically be greater than 0.8 mEq/L, but to avoid
toxicity, the level should not usually exceed 1.5 mEq/L. It is important to
know what other medications a pa-tient may be taking, because many drugs
interact with lithium and can lead to increased or decreased lithium levels and
possibly adverse effects (Table 78.2).
To reach
therapeutic levels rapidly in healthy younger pa-tients with normal renal and
cardiac function, the psychiatrist may prescribe 300 mg of lithium carbonate
four times daily from the outset, sampling the first plasma level after 5 days
(or sooner should toxic signs become apparent). Thereafter, the dose should be
adjusted to achieve a 12-hour plasma concentration between 0.8 and 1.3 mEq/L at
steady state.
In a
patient with mild hypomanic symptoms, by contrast, it may be wiser to begin
with a lower lithium dose, such as 300 mg b.i.d., taking longer to achieve
therapeutic levels but, at the same time, minimizing side effects that could
trouble the patient and hamper cooperation. Once steady state has been achieved
at thera-peutic concentrations and the patient is clinically stable, lithium
can be administered to most patients in a once-daily dose, usually at bedtime.
Not only is this schedule easier to remember, but it tends to decrease such
common side effects as tremor and polyuria.
The most
common acute adverse effects from lithium are nausea, vomiting, diarrhea,
postural tremor, polydipsia and polyuria (Table 78.2). If troublesome, these
can usually be miti-gated by a slower dosage increase or other measures. More
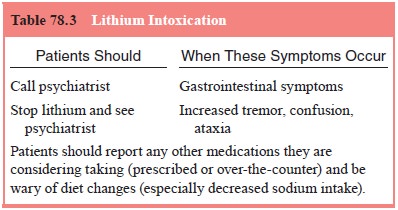
severe symptoms and signs, including confusion and ataxia, may herald lithium
intoxication and should prompt an immediate blood as-say and, if necessary,
temporary discontinuation or dosage re-duction (see Table 78.3).
Anxiolytic Agents
Among
current anxiolytic agents, benzodiazepines are usually se-lected as adjuncts to
treat acute mania because of their safety and efficacy. Benzodiazepines have a
wide margin of safety and can be safely administered in even very high doses,
suppressing potentially dangerous excitement and allowing patients much needed
sleep. When used together with an antipsychotic agent, benzodiazepines
counteract the antipsychotic agent’s tendency to provoke extrapy-ramidal
reactions and seizures. For lorazepam, 1 to 2 mg can be administered by mouth
or intramuscularly as frequently as hourly.
Valproate
Valproate
is available in the USA as valproic acid or divalproex sodium, a compound
containing equal parts valproic acid and sodium valproate. Divalproex is better
tolerated than valproic acid, has been studied more extensively and is more
commonly
used. All
valproate preparations are rapidly absorbed after oral administration, reaching
peak plasma levels within 2 to 4 hours of ingestion. Food may delay absorption
but does not affect bio-availability. Valproate is rapidly distributed and
highly bound (90%) to plasma proteins. Its half-life ranges from 9 to 16 hours,
depending on whether it is taken alone or with other medications, and it takes
1 to 4 days to attain steady state.
Experts
usually rank lithium as the treatment of choice for a patient with classic
mania, but divalproex is an accept-able first-line alternative. It may be used
singly in patients who cannot tolerate lithium. For patients who do not respond
to lithium, there are no secure data on whether divalproex should be added as
an adjunct or substituted, but many psychiatrists would choose the former in a
patient who appears to respond at least partially to lithium and the latter in
patients for whom lithium seems to afford no benefit. Increasingly,
psychiatrists are turning to divalproex first for manic patients with organic
brain impairment, rapid cycling, mixed or dysphoric mania, or comorbid
substance abuse (Bowden et al., 1994;
Calabrese et al., 1993).
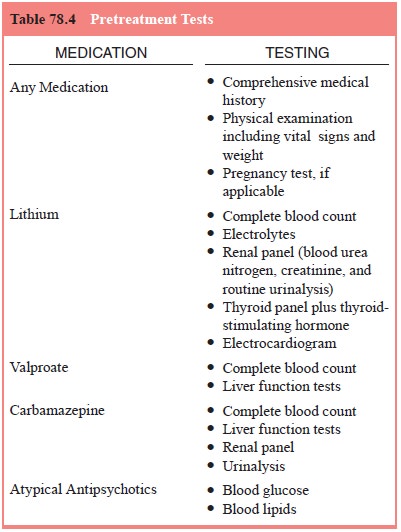
Before
initiating divalproex, the psychiatrist should obtain a comprehensive medical
history and insure that a physical exam-ination has been performed, with
particular attention to sugges-tions of liver disease or bleeding abnormalities
(see Table 78.4).
Baseline
liver and hematological functions are measured before treatment, every 1 to 4
weeks for the first 6 months, and then every 3 to 6 months. Evidence of
hemorrhage, bruising, or a dis-order of hemostasis–coagulation suggests a
reduction of dosage or withdrawal of therapy. The drug should be discontinued
im-mediately in the presence of significant hepatic dysfunction.
A typical
starting dose for healthy adults is 750 mg/day in divided doses. The dose can
then be adjusted to achieve a 12-hour serum valproate concentration between 50
and 125 µg/mL. The time of dosing is
determined by possible side effects and, if tolerated, once-a-day dosing can be
employed. As with lith-ium, the antimanic response to valproate typically
occurs after 1 to 2 weeks.
Adverse
effects that appear early in the course of therapy are usually mild and
transient, and tend to resolve in time. Gas-trointestinal upset is probably the
most common complaint in patients taking valproate and tends to be less of a
problem with the enteric-coated divalproex sodium preparation. The
adminis-tration of a histamine H2 antagonist such as famotidine (Pepcid) or
cimetidine (Tagamet) may alleviate persistent gastrointestinal problems (Stoll et al., 1991). Other common complaints
include tremor, sedation, increased appetite and weight, and alopecia. Weight
gain is even more of a problem when other drugs are ad-ministered that also
promote weight gain, such as lithium, antip-sychotic and other antiepileptics.
Less common are ataxia, rashes and hematological dysfunction, such as
thrombocytopenia and platelet dysfunction. Platelet count usually recovers with
a dosage decrease, but the occurrence of thrombocytopenia or leukopenia may
necessitate the discontinuation of valproate. Serum hepatic transaminase
elevations are common, dose related, and usually self-limiting and benign.
Fatal hepatotoxicity is extremely rare, is usually restricted to young
children, and usually develops within the first 6 months of valproate therapy.
Other serious problems include pancreatitis and teratogenesis (McElroy et al., 1989). There is also a concern
that polycystic ovary syndrome, possibly associated with weight gain, may be a
risk for young women who take valproate. If at any point during administration
the side ef-fects of valproate become intolerable, the psychiatrist may need to
discontinue it and try one of the other treatments described in this section as
an alternative. If valproate is tolerated but not totally effective, the
psychiatrist might use one of the other treat-ments as an adjunct.
Carbamazepine
In light
of the less well-substantiated evidence for the efficacy of carbamazepine, we
place this anticonvulsant fourth in the antimania algorithm – behind lithium,
valproate, and olanzap-ine. The decision to move on to carbamazepine – and
whether to use it alone or in addition to lithium, valproate, or olanzapine –
hinges on the same treatment considerations listed earlier. If a patient has
been treated with one or more of these agents in a previous manic episode, that
experience may guide treatment of a
current episode.
Carbamazepine’s
absorption and metabolism are variable. Peak plasma levels occur within 4 to 6
hours after ingestion of the solid dosage form. Bioavailability is estimated at
85% but may be less when the drug is taken with meals. Eighty percent of plasma
carbamazepine is protein bound, and its half-life ranges from 5 to 26 hours
(after 3–4 weeks of treatment). Carbamazepine’s active metabolite,
10,11-epoxide, has a half-life of about 6 hours.
Carbamazepine
is metabolized by the hepatic cytochrome P450 2D6 system. It causes an
induction of the cytochrome P450 enzymes, often resulting in an increased rate
of its own metabolism over several weeks, as well as that of other drugs
metabolized by the P450 system (Table 78.5). Because of enzyme induction, the
dose of carbamazepine often must be raised after 2 to 4 months of treatment.
Steady state may be attained within 4 to 5 days, but when clearance increases
as a result of autoinduction, steady state may not be achieved for 3 to 4
weeks. Concomitant administration of drugs that inhibit P450 (see Table 78.6)
will increase plasma levels of carbamazepine. Conversely, drugs that induce
P450 enzymes – such as phenobarbital, phenytoin, or primidone – can decrease
carbamazepine levels. Before carbamazepine is started, baseline blood and
platelet counts, urinalysis, and liver and kidney function tests are in order
(see Table 78.4). Although earlier guidelines called for routine monitoring of
some or all of these indices, and some psychiatrists still obtain blood counts
once or twice during the first few months of treatment and when plasma
concentrations are sampled, a more general consensus at present is to instruct
patients and family members to contact the psychiatrist immediately if
petechiae, pallor, weakness, fever, or infection occur, at which time the
psychiatrist should order relevant tests.
Used as a
monotherapy, the typical starting dose for carbamazepine is 200 to 400 mg/day
in three or four divided doses, increased to 800 to 1000 mg/day by the end of
the first week. If clinical improvement is insufficient by the end of the
second week, and the patient has not had intolerable side effects to the drug,
increases to as high as 1600 mg/day may be considered. Although there are no
good studies of the correlation between blood level and clinical response, a
common target range for ma-nia is 4 to 15 ng/mL. If carbamazepine is combined
with lithium
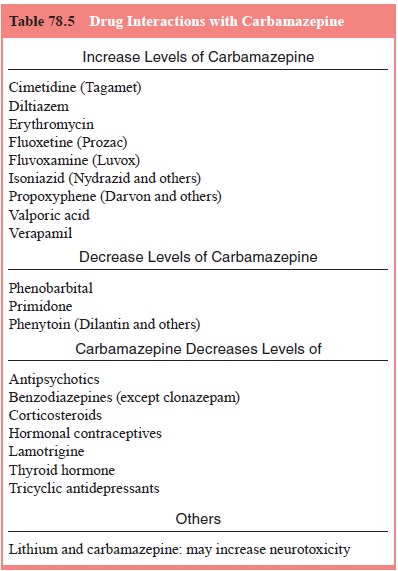
or
neuroleptics, lower doses and blood levels of carbamazepine are often used. If
valproate and carbamazepine are administered simultaneously, blood levels of
each should be monitored care-fully because of complex interactions between the
two agents.
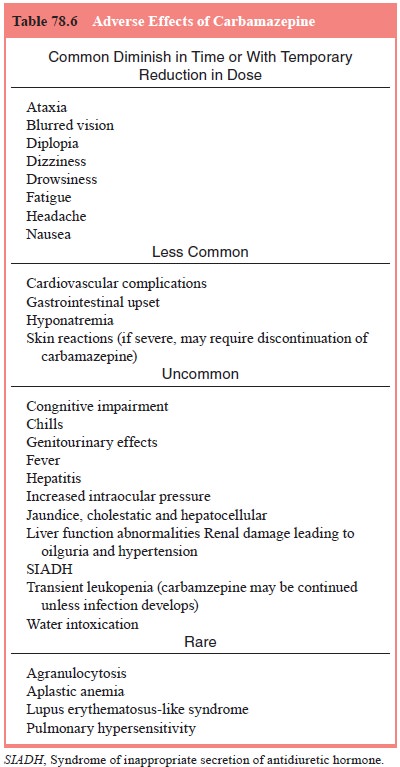
When the
dose of carbamazepine is built up rapidly, side effects are more likely. The
most common effects in the first cou-ple of weeks are drowsiness, dizziness,
ataxia, diplopia, nausea, blurred vision and fatigue (Table 78.6). These tend
to diminish in time or to respond to a temporary reduction in dose. Less
com-mon reactions include gastrointestinal upset, hyponatremia and a variety of
skin reactions, some of which are severe enough to require discontinuation of
carbamazepine. About 10% of patients experience transient leukopenia, but
unless infection develops, carbamazepine may be continued. More serious
hematopoietic re-actions, including aplastic anemia and agranulocytosis, are
rare.
Other Drug Treatments
Since
carbamazepine and valproate are efficacious for acute ma-nia, new antiepileptic
medications are often tested for mania. Lamotrigine, which appears useful in
maintenance, bipolar de-pression and rapid-cycling (see below), has not shown
efficacy in acute mania, with the exception of one recent double-blind trial
(Berk, 1999; Anand et al., 1999;
Bowden et al., 2000).
Nonpharmacotherapy: Electroconvulsive Therapy
When an acutely manic patient is unresponsive to or intolerant of medication, or if medication presents other risks (e.g., during preg-nancy), ECT should be seriously considered and may be lifesaving. Although there are no coherent theories about why ECT is effective in acute mania, it has been used for more than half a century, and there are widespread clinical impressions of its safety and efficacy.
Related Topics