Chapter: Essentials of Psychiatry: Antipsychotic Drugs
Treatment of Different Phases of Schizophrenia
Treatment
of Different Phases of Schizophrenia
Treatment During the Acute Phase
Route of Administration
In
clinical situations, antipsychotic medications can be adminis-tered in oral
forms, including oral tablets, oral liquid concentrates and orally dissolving
formulations, as short-acting intramuscular preparations, or as long-acting
depot preparations (American Psychiatric Association, 2000). In most cases,
patients who are cooperative prefer oral administration to parenteral medications.
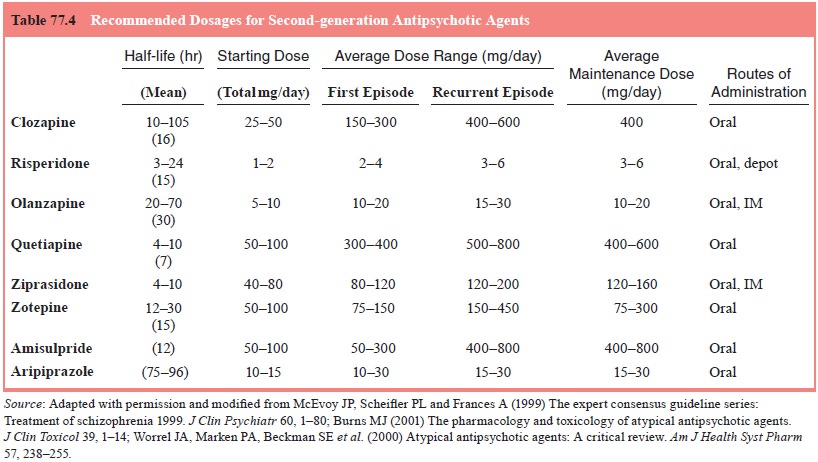
A summary of dosing recommendations for newer agents is pro-vided in Table
77.4. Oral antipsychotic medications tend to be rapidly and well absorbed from
the gastrointestinal tract, and reach a peak plasma concentration in 1 to 10
hours (Burns, 2001). The long average half-life (12–24 hours) and active
metabolites of most oral antipsychotic drugs allow for once- to twice-daily
dos-ing (Marder, 1997; Burns, 2001). Among the second-generation agents,
quetiapine and ziprasidone have relatively shorter half-lives (Table 77.4), and
should be administered in divided doses (Markowitz et al., 1999). A single or twice-daily dose of an oral preparation
will result in steady-state blood levels in 2 to 5 days (Dahl, 1990).
Short-acting
intramuscular (IM) medications are particu-larly useful in the management of
acute pathologic excitement and agitation (Buckley, 1999). The main indication
for the use of a short-acting parenteral form in the acute situation is to
treat severely disturbed patients who cannot be verbally redirected, who may be
violent, and who may have to be medicated over objection. Short-acting IM
preparations can reach a peak con-centration 30 to 60 minutes after the
medication is administered (Dahl, 1990).
Selection of an Antipsychotic Agent
Selection of an agent in emergency settings for the management of the gross agitation, excitement and violent behavior associated with psychosis might be based on clinical symptoms, differences in efficacy or side effects of candidate drugs, or, more pragmati-cally, the formulation of a drug as it affects route of administra-tion, onset and duration (Hirsch and Barnes, 1995; Allen, 2000). Most agitated patients will assent to oral medication and, in a survey of 51 psychiatric emergency services, the medical doctors estimated that only 10% of emergency patients require injectable medications (Currier, 2000). Practice and legal requirements concerning injectable medications differ substantially across the globe. The US Health Care Finance Administration’s (HCFA) regulations regarding so-called “chemical restraint” call for it to be a last resort, which would suggest that oral medication should be offered whenever it is possible to speak with the patient (Allen, 2000). Intramuscular treatments, however, remain nec-essary for some agitated or aggressive patients who refuse oral medications of any kind (Allen et al., 2001). In these situations, many clinicians avoid high doses on antipsychotic medications in favor of a combination of an antipsychotic and a benzodiazepine (Miyamoto et al., 2002b).
Dose of Antipsychotic Agent
Effective Doses of First-generation Antipsychotics The goal of pharmacotherapy is to maximize
efficacy and minimize ad-verse effects with the lowest effective dose. When
groups of pa-tients are assigned to higher doses, such as more than 2000 mg
chlorpromazine or 40 mg haloperidol equivalent, the rate and amount of
improvement are no greater than for those assigned to more moderate doses
(Marder, 1996).
Effective Doses of Second-generation Antipsychotics
The dosage recommendations for
second-generation antipsychotic drugs are summarized in Table 77.4. Although
clinical trial data show that second-generation antipsychotics are efficacious
and cause fewer EPS than the conventional agents, optimal dosing constitutes a
critical issue in their effective use.
Treatment Resistance
Patients
with schizophrenia may manifest poor response to treatment because of
intolerance to medication, poor compliance, inappropriate dosing, as well as
true resistance of their illness to antipsychotic medications. It has been
consistently reported
that
approximately 10 to 15% of patients with first-episode schizophrenia are
resistant to drug treatment (Lieberman et
al., 1993), and between 30 to 60% of patients become only partially
responsive or completely unresponsive to treatment during the course of the
illness (Davis and Casper, 1977; Essock et
al., 1996; Lieberman, 1999). Before a patient is considered
treatment-resistant, an optimized medication and treatment trial should be
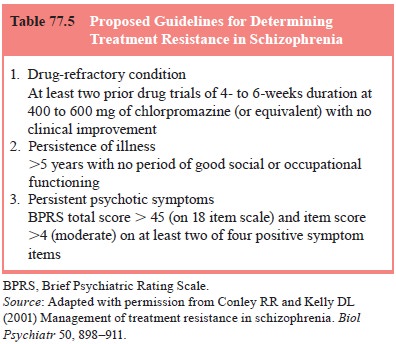
employed (Conley and Kelly, 2001). The most accepted criteria for defining
treatment resistance in schizophrenia were initially utilized by Kane and
collaborators (1988) in the Multicenter Clozapine Trial (MCT), and the modified
criteria have been used to define treatment resistance (Table 77.5). Although
most definitions of treatment resistance focus on the persistence of positive
symptoms, there is growing awareness of the problems of persistent negative
symptoms and cognitive impairments, which may have an important impact on level
of functioning, psychosocial integration and quality of life (Conley and Kelly,
2001; Peuskens, 1999).
Only
clozapine has consistently demonstrated efficacy for psychotic symptoms in
well-defined treatment refractory patients. The mechanism responsible for this
therapeutic ad-vantage remains uncertain. Thus, clozapine remains the “gold
standard” for treatment of this patient population. The evidence is strongest
in support of clozapine monotherapy as an interven-tion for treatment-resistant
patients; serum levels of 350 µg/mL or
greater have been associated with maximal likelihood of re-sponse (Miller,
1996).
Since the
approval of clozapine, attention has shifted to a greater focus on the use of
other second-generation antipsychot-ics for managing treatment resistance in
schizophrenia, but the relative efficacy of other second-generation
antipsychotics is less clear.
Given the
risk of agranulocytosis, the burden of side ef-fects and the requirement of
white blood cell monitoring, the second-generation agents (risperidone,
olanzapine, quetiapine and ziprasidone) should be tried before proceeding to
clozapine in almost all patients (Conley and Kelly, 2001; Miyamoto et al., 2002a). Many clinicians express
the impression that certain pa-tients do respond preferentially to a single
agent of this class. Sequential controlled trials of the newer agents in
treatment-resistant patients will be necessary fully to examine this issue.
Treatment During the Resolving Phase
During
the resolving phase, the goals of treatment are to minimize stress on the
patient, to facilitate the patient’s return to community life, and to establish
a long-term maintenance plan (Marder, 1999; American Psychiatric Association,
2000). If a particular antipsy-chotic medication has improved the acute
symptoms, it should be continued at the same dose for the next 6 months, before
a lower maintenance dose is considered for continued treatment (American
Psychiatric Association, 2000). Rapid dose reduction or discontin-uation of the
medications during the resolving phase may result in relatively rapid relapse
(American Psychiatric Association, 2000). If the psychiatrist has decided to
switch the therapy to a long-acting depot antipsychotic agent, this can often
be accompanied during this phase. This may also be a reasonable time to educate
the patient and family regarding the course and outcome of schizo-phrenia, as
well as factors that influence the outcome such as drug compliance (Marder,
1997; American Psychiatric Association, 2000). Patients should be helped to
begin formulating a rehabilita-tion plan through realistic goal setting
(Marder, 1997).
Treatment During the Stable Phase (Maintenance Treatment)
The goals
of treatment during the stable or maintenance phase are to maintain symptom
remission, to prevent psychotic relapse, to implement a plan for rehabilitation
and to improve the patient’s quality of life (Marder, 1999; American
Psychiatric Associations, 2000). Current guidelines recommend that
first-episode patients should be treated for 1 to 2 years; however, 75% of
patients will have relapses after their treatment is discontinued (Kissling,
1991; Davis et al., 1994; Lehman and
Steinwachs, 1998; Ameri-can Psychiatric Association, 2000). Patients who have
had mul-tiple episodes should receive at least 5 years of maintenance therapy
(Kissling, 1991; Davis et al., 1994;
American Psychiatric Association, 2000). Patients with severe or dangerous
episodes should probably be treated indefinitely (American Psychiatric
Association, 2000).
For
conventional antipsychotics, the risk of long-term side effects such as the
development of TD inspired a search for strategies to reduce patients’ exposure
to these agents, and strate-gies for preventing relapse during the maintenance
phase has fo-cused on finding dosages that minimize drug adverse effects and
provide adequate protection against psychotic relapse (Marder, 1999).
Maintenance studies of the dose–response relationship found that lowering the
dose prescribed for acute treatment by about 80% may be relatively safe for
maintenance, although re-lapse rates are excessively high when doses are
reduced to about 10% of an acute dose (Marder et al., 1987; Hogarty et al.,
1988; Kane et al., 1983). The
international consensus conference rec-ommended a gradual reduction in
antipsychotic dose of approxi-mately 20% every 6 months until a minimal
maintenance dose is reached (Kissling, 1991).
Related Topics