Chapter: Modern Pharmacology with Clinical Applications: Antineoplastic Agents
Antimetabolites: Purine Analogues
Purine Analogues
Thioguanine (6-Thioguanine)
Thioguanine is an analogue of
the natural purine gua-nine in which a hydroxyl group has been replaced by a
sulfhydryl group in the 6-position. Two major mecha-nisms of cytotoxicity have
been proposed for 6-thiogua-nine: (1) incorporation of the thio nucleotide
analogue into DNA or RNA and (2) feedback inhibition of purine nucleotide
synthesis. Both of these actions require initial activation of the drug by the
enzyme hypoxanthine guanine–phosphoribosyltransferase (HGPRTase), as follows:
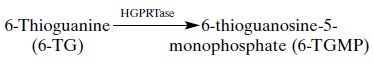
The product of this reaction,
6-TGMP, can eventu-ally be converted to deoxy-6-thioguanosine-triphos-phate
(dTGTP), which has been shown to be incorpo-rated into DNA. Resistance of human leukemia cells to
thioguanine has been correlated with decreased activity of HGPRTase and to
increased inactivation of the thio nucleotides by alkaline phosphatase.
Thioguanine is slowly
absorbed after oral adminis-tration; parent drug levels are barely detectable,
and peak levels of metabolites occur only after 6 to 8 hours. Total urinary
excretion of metabolites in the first 24 hours is 24 to 46% of the administered
dose.
Thioguanine is used primarily
as part of a combined induction of chemotherapy in acute myelogenous leukemia.
Myelosuppression, with
leukopenia and thrombocy-topenia appearing 7 to 10 days after treatment, and
mild nausea are the most common adverse effects. Liver toxi-city with jaundice
has been reported in some patients but appears to be less common than with
mercaptopurine.
Mercaptopurine (6-Mercaptopurine)
Mercaptopurine (Purinethol) is an analogue of
hypo-xanthine and was one of the first agents shown to be ac-tive against acute
leukemias. It is now used as part of maintenance therapy in acute lymphoblastic
leukemia. Mercaptopurine must be activated to a nucleotide by the enzyme
HGPRTase. This metabolite is capable of inhibiting the synthesis of the normal
purines adenine and guanine at the initial aminotransferase step and
in-hibiting the conversion of inosinic acid to the nu-cleotides adenylate and
guanylate at several steps. Some mercaptopurine is also incorporated into DNA
in the form of thioguanine. The relative significance of these mechanisms to
the antitumor action of mercap-topurine is not clear.
Resistance to mercaptopurine may be a result of de-creased drug activation by
HGPRTase or increased in-activation by alkaline phosphatase.
The plasma half-life of an
intravenous bolus injec-tion of mercaptopurine is 21 minutes in children and 47
minutes in adults. After oral administration, peak plasma levels are attained
within 2 hours. The drug is 20% bound to plasma proteins and does not enter the
CSF. Xanthine oxidase is the primary enzyme involved in the metabolic inactivation
of mercaptopurine.
Mercaptopurine is used in the
maintenance therapy of acute lymphoblastic leukemia. It also displays activ-ity
against acute and chronic myelogenous leukemias.
The major toxicities of
mercaptopurine are myelo-suppression, nausea, vomiting, and hepatic toxicity.
Fludarabine
Fludarabine (Fludara) is a fluorinated purine
analogue of the antiviral agent vidarabine. The active metabolite, 2-fluoro-ara-adenosine
triphosphate, inhibits various enzymes involved in DNA synthesis, including DNA
polymerase- , ribonucleotide reductase, and DNA pri-mase. Unlike most
antimetabolites, it is toxic to nonpro-liferating as well as dividing cells,
primarily lymphocytes and lymphoid cancer cells.
The drug is highly active in
the treatment of chronic lymphocytic leukemia, with approximately 40% of
pa-tients achieving remissions after previous therapy with alkylating agents
has failed. Activity is also seen in the low-grade lymphomas.
The major side effect is
myelosuppression, which contributes to fevers and infections in as many as half
of treated patients. Nausea and vomiting are mild. Occasional neurotoxicity has
been noted at higher doses, with agitation, confusion, and visual disturbances.
Pentostatin
Pentostatin (Nipent, deoxycoformycin) is a purine
iso-lated from fermentation cultures of the microbe Streptomyces antibioticus. Its mechanism of action in-volves
inhibition of the enzyme adenosine deaminase, which plays an important role in
purine salvage path-ways and DNA synthesis. The resulting accumulation of
deoxyadenosine triphosphate (dATP) is highly toxic to lymphocytes.
Pentostatin is effective in
the therapy of hairy cell leukemia, producing remissions in 80 to 90% of
patients and complete remissions in more than 50%. The major toxic effects of
the drug include myelosuppression, nau-sea, and skin rashes.
Cladribine
Cladribine (Leustatin) is a synthetic purine
nucleoside that is converted to an active cytotoxic metabolite by the enzyme
deoxycytidine kinase. Like the other purine antimetabolites, it is relatively
selective for both normal and malignant lymphoid cells and kills resting as
well as dividing cells by mechanisms that are not completely understood.
The drug is highly active
against hairy cell leukemia, producing complete remissions in more than 60% of
pa-tients treated with a single 7-day course. Activity has also been noted in
other low-grade lymphoid malignan-cies. The major side effect is
myelosuppression.
Related Topics