Chapter: Modern Pharmacology with Clinical Applications: Antineoplastic Agents
Alkylating Agents
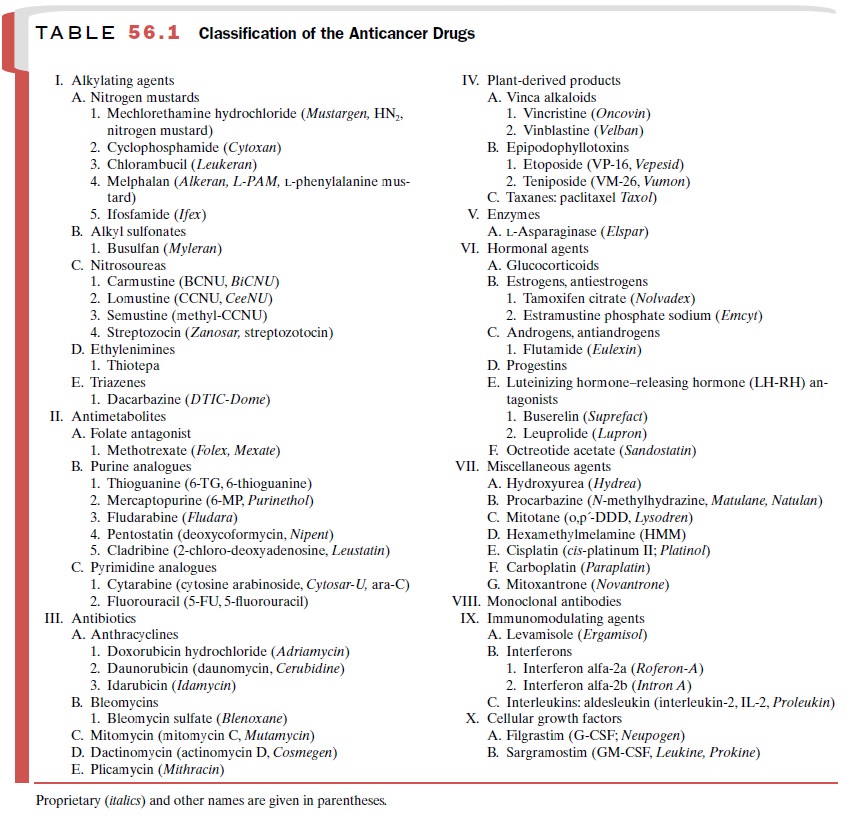
ALKYLATING
AGENTS
The alkylating agents are the
largest class of anticancer agents, comprising five subgroups: nitrogen
mustards, alkyl sulfonates, nitrosoureas, ethyleneimine, and thi-azines (Table
56.1). Several other agents, including pro-carbazine, hexamethylmelamine,
dacarbazine, estra-mustine, and mitomycin C, are thought to act at least in
part by alkylation.
By definition, alkylating agents are compounds that are
capable of introducing alkyl groups into nucle-ophilic sites on other molecules
through the formation of covalent bonds.
These nucleophilic targets for alkyla-tion include the sulfhydryl, amino,
phosphate, hydroxyl, carboxyl, and imidazole groups that are present in
macromolecules and low-molecular-weight compounds within cells.
The macromolecular sites of alkylation damage in-clude DNA, RNA, and various enzymes. The inhibition of DNA synthesis occurs at drug concentrations that are lower than those required to inhibit RNA and protein synthesis, and the degree of DNA alkylation correlates especially well with the cytotoxicity of these drugs. This interaction also accounts for the mutagenic and car-cinogenic properties of the alkylating agents. The reac-tions of various alkylating agents with DNA have been studied in detail, and the 7-nitrogen (N7) and 6-oxygen (O6) of guanine have been shown to be particularly susceptible to attack by electrophilic compounds. There are several possible consequences of N7 guanine alkyl-ation:
·
Cross-linkage. Bifunctional alkylating
agents, such as the nitrogen
mustards, may form cova-lent bonds with each of two adjacent guanine residues.
Such interstrand cross-linkages will inhibit
DNA replication and transcription. Intrastrand cross-links also may be
produced between DNA and a nearby protein.
·
Mispairing of bases. Alkylating at N7 changes the O6 of guanine to its enol tautomer,
which can then form base pairs with thymine. This may lead to gene miscoding,
with adenine– thymine pairs replacing guanine–cytosine. The result is the
production of defective proteins.
·
Depurination. N7 alkylation may cause
cleav-age of the imidazole ring and excision of the guanine residue, leading to
DNA strand break-age.
Although all alkylating
agents can cause the kinds of genetic damage just discussed, individual drugs
differ from one another in their electrophilic reactivity, the structure of
their reactive intermediates, and their phar-macokinetic properties. These
differences will be re-flected in the spectrum of their antitumor activities
and in the toxicities they produce in normal tissues.
Related Topics