Chapter: Basic & Clinical Pharmacology : Dermatologic Pharmacology
Anti Inflammatory Agents
ANTI INFLAMMATORY AGENTS
TOPICAL CORTICOSTEROIDS
The remarkable
efficacy of topical corticosteroids in the treatment of inflammatory dermatoses
was noted soon after the introduction of hydrocortisone in 1952. Numerous
analogs are now availablethat offer extensive choices of potencies,
concentrations, and vehicles. The therapeutic effectiveness of topical
corticosteroids is based primarily on their anti-inflammatory activity.
Definitive explanations of the effects of corticosteroids on endogenous
mediators of inflammation await further experimental clarifica-tion. The
antimitotic effects of corticosteroids on human epider-mis may account for an
additional mechanism of action in psoriasis and other dermatologic diseases
associated with increased cell turnover.
Chemistry & Pharmacokinetics
The original topical
glucocorticosteroid was hydrocortisone, the natural glucocorticosteroid of the
adrenal cortex. The 9α-fluoro derivative of hydrocortisone was
active topically, but its salt-retaining properties made it undesirable even
for topical use. Prednisolone and methylprednisolone are as active topically as
hydrocortisone (Table 61–2). The 9α-fluorinated steroids dexam-ethasone and
betamethasone did not have any advantage over hydrocortisone. However,
triamcinolone and fluocinolone, the acetonide derivatives of the fluorinated
steroids, do have a distinct efficacy advantage in topical therapy. Similarly,
betamethasone is not very active topically, but attaching a 5-carbon valerate
chain to the 17-hydroxyl position results in a compound over 300 times as
active as hydrocortisone for topical use. Fluocinonide is the 21-acetate
derivative of fluocinolone acetonide; the addition of the 21-acetate enhances
the topical activity about five-fold. Fluorination of the corticoid is not
required for high potency.Corticosteroids are only minimally absorbed following
applica-tion to normal skin; for example, approximately 1% of a dose of
hydrocortisone solution applied to the ventral forearm is absorbed. Long-term
occlusion with an impermeable film such as plastic wrap is an effective method
of enhancing penetration, yielding a tenfold increase in absorption. There is a
marked regional ana-tomic variation in corticosteroid penetration. Compared
with the absorption from the forearm, hydrocortisone is absorbed 0.14 times as
well through the plantar foot arch, 0.83 times as well through the palm, 3.5
times as well through the scalp, 6 times as well through the forehead, 9 times
as well through vulvar skin, and 42 times as well through scrotal skin. Penetration
is increased severalfold in the inflamed skin of atopic dermatitis; and in
severe exfoliative diseases, such as erythrodermic psoriasis, there appears to
be little barrier to penetration.
Experimental studies on the percutaneous absorption of hydro-cortisone fail to reveal a significant increase in absorption when applied on a repetitive basis and a single daily application may be effective in most conditions. Ointment bases tend to give better activity to the corticosteroid than do cream or lotion vehicles. Increasing the concentration of a corticosteroid increases the pen-etration but not proportionately. For example, approximately 1% of a 0.25% hydrocortisone solution is absorbed from the forearm. A 10-fold increase in concentration causes only a fourfold increase in absorption. Solubility of the corticosteroid in the vehicle is a significant determinant of the percutaneous absorption of a topi-cal steroid. Marked increases in efficacy are noted when optimized vehicles are used, as demonstrated by newer formulations of betamethasone dipropionate and diflorasone diacetate.
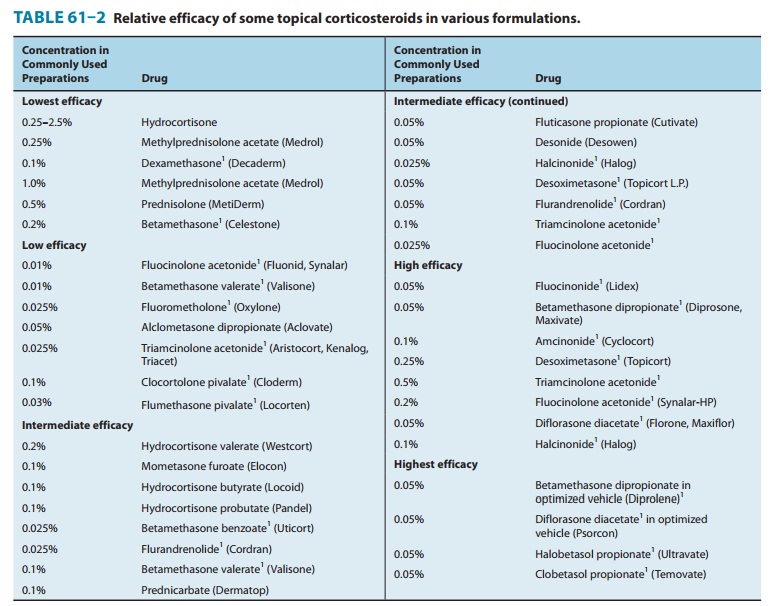
Table
61–2 groups topical corticosteroid formulations accord-ing to approximate
relative efficacy. Table 61–3 lists major derma-tologic diseases in order of
their responsiveness to these drugs. In the first group of diseases, low- to
medium-efficacy corticosteroid preparations often produce clinical remission.
In the second group, it is often necessary to use high-efficacy preparations,
occlusion therapy, or both. Once a remission has been achieved, every effort
should be made to maintain the improvement with a low-efficacy corticosteroid.
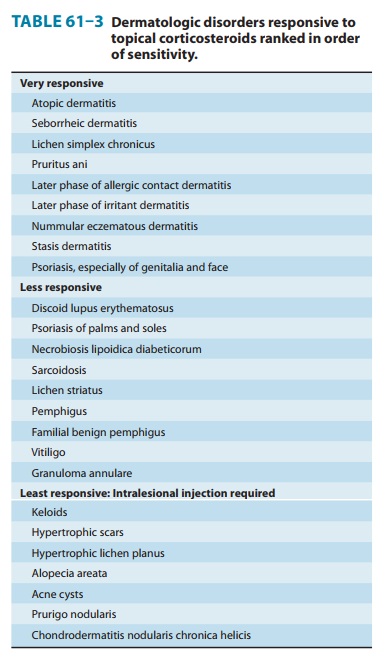
The limited
penetration of topical corticosteroids can be over-come in certain clinical
circumstances by the intralesional injec-tion of relatively insoluble
corticosteroids, eg, triamcinolone acetonide, triamcinolone diacetate,
triamcinolone hexacetonide,and betamethasone acetate-phosphate. When these
agents are injected into the lesion, measurable amounts remain in place and are
gradually released for 3–4 weeks. This form of therapy is often effective for
the lesions listed in Table 61–3 that are generally unresponsive to topical
corticosteroids. The dosage of the triam-cinolone salts should be limited to 1
mg per treatment site, ie, 0.1 mL of 10 mg/mL suspension, to decrease the
incidence of local atrophy .
Adverse Effects
All absorbable topical
corticosteroids possess the potential to sup-press the pituitary-adrenal axis .
Although most patients with pituitary-adrenal axis suppression demonstrate only
a laboratory test abnormality, cases of severely impaired stress
Iatrogenic Cushing’s syndrome may occur as a result of protracted use of topical
corticosteroids in large quanti-ties. Applying potent corticosteroids to
extensive areas of the body for prolonged periods, with or without occlusion,
increases the likelihood of systemic effects. Fewer of these factors are
required to produce adverse systemic effects in children, and growth
retar-dation is of particular concern in the pediatric age group.
Adverse local effects
of topical corticosteroids include the fol-lowing: atrophy, which may present
as depressed, shiny, often wrinkled “cigarette paper”-appearing skin with
prominent telang-iectases and a tendency to develop purpura and ecchymosis;
corti-coid rosacea, with persistent erythema, telangiectatic vessels, pustules,
and papules in central facial distribution; perioral derma-titis, steroid acne,
alterations of cutaneous infections, hypopig-mentation, hypertrichosis;
increased intraocular pressure; and allergic contact dermatitis. The latter may
be confirmed by patch
testing
with high concentrations of corticosteroids, ie, 1% in pet-rolatum, because
topical corticosteroids are not irritating. Screening for allergic contact
dermatitis potential is performed with tixocor-tol pivalate, budesonide, and
hydrocortisone valerate or butyrate. Topical corticosteroids are
contraindicated in individuals who demonstrate hypersensitivity to them. Some
sensitized subjects develop a generalized flare when dosed with
adrenocorticotropic hormone or oral prednisone.
TAR COMPOUNDS
Tar
preparations are used mainly in the treatment of psoriasis, dermatitis, and lichen
simplex chronicus. The phenolic constitu-ents endow these compounds with
antipruritic properties, making them particularly valuable in the treatment of
chronic lichenified dermatitis. Acute dermatitis with vesiculation and oozing
may be irritated by even weak tar preparations, which should be avoided.
However, in the subacute and chronic stages of dermatitis and psoriasis, these
preparations are quite useful and offer an alterna-tive to the use of topical
corticosteroids.
The most common
adverse reaction to coal tar compounds is an irritant folliculitis,
necessitating discontinuance of therapy to the affected areas for a period of
3–5 days. Photoirritation and allergic contact dermatitis may also occur. Tar
preparations should be avoided in patients who have previously exhibited
sensitivity to them.
Related Topics