Chapter: Clinical Anesthesiology: Anesthetic Management: Anesthesia for Patients with Endocrine Disease
Anesthesia for Diabetes Mellitus
DIABETES MELLITUS
Clinical Manifestations
Diabetes mellitus is characterized by impairment of carbohydrate
metabolism caused by an absolute or relative deficiency of insulin or of
insulin respon-siveness, which leads to hyperglycemia and glycos-uria. The
diagnosis is based on an elevated fasting plasma glucose greater than 126 mg/dL
or glycated hemoglobin (HbA1c) of 6.5% or greater. Values
are sometimes reported for blood glucose, which runs 12–15% lower than plasma
glucose. Even when test-ing whole blood, newer glucose meters calculate and
display plasma glucose.
Diabetes is classified in multiple ways (Table 34–2). Type 1 (insulin-requiring
due to endogenous insulin deficiency) and type 2 (insulin-resistant) diabetes
are the most common and well

known. Diabetic ketoacidosis (DKA) is associated with type 1 diabetes
mellitus, but rarely individuals with DKA appear phenotypically to have type 2
dia-betes mellitus. Long-term complications of diabetes include retinopathy,
kidney disease, hypertension, coronary artery disease, peripheral and cerebral
vascular disease, and peripheral and autonomic neuropathies.
There are three life-threatening acute compli-cations of diabetes and its treatment—DKA, hyper-osmolar nonketotic coma, and hypoglycemia—in addition to other acute medical problems (such as sepsis) in which the presence of diabetes makes treatment more difficult. Decreased insulin activ-ity allows the catabolism of free fatty acids into ketone bodies (acetoacetate and β-hydroxybutyrate), some of which are weak acids . Accumulation of these organic acids results in DKA, an anion-gap metabolic acidosis. DKA can easily be distinguished from lactic acidosis, with which it can coexist; lactic acidosis is identified by elevated plasma lactate (>6 mmol/L) and the absence of urine and plasma ketones (although they can occur concurrently and starvation ketosis may occur with lactic acidosis). Alcoholic ketoacidosis can follow heavy alcohol consumption (binge drinking) in a nondiabetic patient and may include a normal or slightly elevated blood glucose level. Such patients may also have a disproportionate increase in β-hydroxybutyrate compared with acetoacetate, incontrast to those with DKA.
Infection is a common precipitating cause of
DKA in a known diabetic patient, and DKA may be the reason that a previously
undiagnosed person with type 1 diabetes presents for medical treatment.
Clinical manifestations of DKA include tachypnea (respiratory compensation for
the metabolic aci-dosis), abdominal pain, nausea and vomiting, and changes in
sensorium. The treatment of DKA should include correcting the often substantial
hypovole-mia, the hyperglycemia, and the total body potas-sium deficit. This is
typically accomplished with a continuous infusion of isotonic fluids and
potassium and an insulin infusion.
The goal for decreasing blood glucose in keto-acidosis should be 75–100
mg/dL/h or 10%/h. Therapy generally begins with an intravenous insu-lin
infusion at 0.1 units/kg/h. DKA patients may be resistant to insulin, and the
insulin infusion rate may need to be increased if glucose concentrations do not
decrease. As glucose moves intracellularly, so does potassium. Although this
can quickly lead to a critical level of hypokalemia if not corrected,
overaggressive potassium replacement can lead to an equally life-threatening
hyperkalemia. Potassium and blood glucose should be monitored frequently during
treatment of DKA.
Several liters of 0.9% saline (1–2 L the
first hour, followed by 200–500 mL/h) may be required to cor-rect dehydration
in adult patients. When plasma glucose decreases to 250 mg/dL, an infusion of
D5W should be added to the insulin infusion to decrease the possibility of
hypoglycemia and to provide a con-tinuous source of glucose (with the infused insulin) for eventual normalization of intracellular
metabo-lism. Patients may benefit from precise monitoring of urinary output
during initial treatment of DKA.
Bicarbonate is rarely needed to correct severe acidosis (pH < 7.1) as the acidosis corrects with
volume expansion and with normalization of the plasma glucose concentration.
Ketoacidosis is not a feature of hyperosmolarnonketotic
coma possibly because enough insu-lin is available
to prevent ketone body formation. Instead, a hyperglycemia-induced diuresis
leads to dehydration and hyperosmolality. Severe dehydra-tion may eventually
lead to kidney failure, lactic acidosis, and a predisposition to form
intravascular thromboses. Hyperosmolality (frequently exceed-ing 360 mOsm/L)
induces dehydration of neurons, causing changes in mental status and seizures.
Severe hyperglycemia causes a factitious hypona-tremia: each 100 mg/dL increase
in plasma glucose lowers plasma sodium concentration by 1.6 mEq/L. Treatment
includes fluid resuscitation with normal saline, relatively small doses of
insulin, and potas-sium supplementation.
Hypoglycemia in the diabetic patient is the
result of an absolute or relative excess of insulin relative to carbohydrate
intake and exercise. Furthermore, dia-betic patients are incompletely able to
counter hypo-glycemia despite secreting glucagon or epinephrine
(counterregulatory failure). The dependence of the brain on glucose as an
energy source makes it the organ most susceptible to episodes of hypoglycemia.
If hypoglycemia is not treated, mental status changescan progress from anxiety,
lightheadedness, or con-fusion to convulsions and coma. Systemic
manifes-tations of hypoglycemia result from catecholamine discharge and include
diaphoresis, tachycardia, and nervousness. Most of the signs and symptoms of
hypoglycemia will be masked by general anesthe-sia. Although the lower boundary
of normal plasma glucose levels is ill-defined, medically important
hypoglycemia is present when plasma glucose is less than 50 mg/dL. The
treatment of hypoglycemia in anesthetized or critically ill patients consists
of intra-venous administration of 50% glucose (each milli-liter of 50% glucose
will raise the blood glucose of a 70-kg patient by approximately 2 mg/dL).
Awake patients can be treated orally with fluids containing glucose or sucrose.
Anesthetic Considerations
A. Preoperative
Abnormally elevated hemoglobin A1c
concentrations identify patients who have maintained poor control of blood
glucose over time. These patients may be at greater risk for perioperative
hyperglycemia, peri-operative complications, and adverse outcomes. The
perioperative morbidity of diabetic patients is related to their preexisting
end-organ damage. Unfortunately, one third to one half of patients with type 2
diabetes mellitus may be unaware of their condition.
A preoperative chest radiograph in a diabetic
patient is more likely to uncover cardiac enlargement, pulmonary vascular
congestion, or pleural effusion, but is not routinely indicated. Diabetic
patients also have an increased incidence of ST-segment and T-wave-segment
abnormalities on preoperative elec-trocardiograms (ECGs). Myocardial ischemia
or old infarction may be evident on an ECG despite a nega-tive history.
Diabetic patients with hypertension have a 50% likelihood of coexisting diabetic
autonomicneuropathy (Table
34–3). Reflex dysfunction ofthe autonomic nervous
system may be increased by old age, diabetes of longer than 10 years’ duration,
coronary artery disease, or β- adrenergic blockade.
Diabetic autonomic neuropathy may limit the
patient’s ability to compensate (with tachycar-dia and increased peripheral
resistance) for intravas-cular volume changes and may predispose the patient
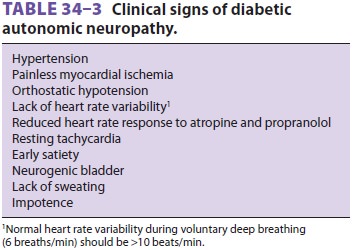
to cardiovascular instability (eg,
postinduction hypotension) and even sudden cardiac death. The incidence of
perioperative cardiovascular instability appears increased by the concomitant
use of angio-tensin-converting enzyme inhibitors or angiotensin receptor
blockers. Autonomic dysfunction contrib-utes to delayed gastric emptying
(diabetic gastropare-sis). Premedication with a nonparticulate antacid and
metoclopramide is often used in an obese diabetic patient with signs of cardiac
autonomic dysfunction. However, autonomic dysfunction can affect the
gas-trointestinal tract without any signs of cardiac involvement.
Diabetic renal dysfunction is manifested first by proteinuria and later
by elevated serum creatinine. By these criteria, most patients with type 1
diabetes have evidence of kidney disease by 30 years of age. Because of an
increased incidence of infections related to a compromised immune system,
strict attention to aseptic technique, important for all patients, is
espe-cially important in those with diabetes.
Chronic hyperglycemia can lead to
glycosyl-ation of tissue proteins and limited mobility of joints.
Temporomandibular joint and cervical spine
mobility should be assessed preoperatively indiabetic patients to reduce the
likelihood of unan-ticipated difficult intubations. Difficult intubation has
been reported in as many as 30% of persons with type 1 diabetes.
B. Intraoperative
The goal of intraoperative blood glucose manage-ment is to avoid
hypoglycemia while maintaining blood glucose below 180
mg/dL. Attempting to maintain strict euglycemia is imprudent; “loose” blood
glucose control (>180 mg/dL) also carries risk. The exact range over which blood glucose
should be maintained in critical illness has been the subject of several much-discussed
clinical trials. Hyperglycemia has been associated with hyperosmo-larity,
infection, poor wound healing, and increased mortality. Severe hyperglycemia
may worsen neu-rological outcome following an episode of cerebral ischemia and
may compromise outcome following cardiac surgery or after an acute myocardial
infarc-tion. Unless severe hyperglycemia is treated aggres-sively in type 1
diabetic patients, metabolic control may be lost, particularly in association
with major surgery or critical illness. Maintaining blood glucose control (<180 mg/dL) in patients
undergoing car-diopulmonary bypass decreases infectious compli-cations. A
benefit of true “tight” control (<150 mg/ dL) during surgery or critical illness has not yet been
demonstrated convincingly and in some studies has been associated with worse
outcome than “looser” control (<180 mg/dL).
Lack of consensus regarding the appropriate target for blood glucose has
not prevented periop-erative glucose management from becoming yet another
indicator of so-called “quality” anesthetic care. Consequently, anesthesia
staff should carefully review their current practices to ensure that their
glucose management protocols are in line with insti-tutional expectations.
Control of blood glucose in pregnant diabetic patients improves fetal
outcome. Nonetheless, as noted earlier, the brain’s dependence on glucose as an
energy supply makes it essential that hypoglyce-mia be avoided.
Th ere are several common perioperative
management regimens for insulin-dependent dia-betic patients. In the most
time-honored (but not terribly effective) approach, the patient receives a
fraction—usually half—of the total morning insu-lin dose in the form of
intermediate-acting insulin (Table 34–4).
To decrease the risk of hypoglycemia, insulin is administered after intravenous access has been
established and the morning blood glucose level is checked. For example, a
patient who nor-mally takes 30 units of NPH (neutral protamine
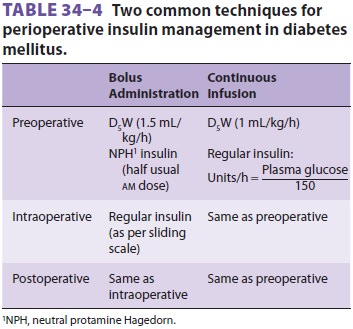
Hagedorn; intermediate-acting) insulin and 10
units of regular or Lispro (short-acting) insulin or insulin analogue each
morning and whose blood glucose is at least 150 mg/dL would receive 15 units
(half the normal 30-unit morning dose) of NPH subcu-taneously before surgery
along with an infusion of 5% dextrose solution (1.5 mL/kg/h). Absorption of
subcutaneous or intramuscular insulin depends on tissue blood flow, however,
and can be unpredict-able during surgery. Dedication of a small-gauge
intravenous line for the dextrose infusion prevents interference with other
intraoperative fluids and drugs. Supplemental dextrose can be administered if
the patient becomes hypoglycemic (<100 mg/dL). However, intraoperative
hyperglycemia (>150–180 mg/dL) is treated with intravenous regular insulin according to
a sliding scale. One unit of regular insu-lin given to an adult usually lowers
plasma glucose by 25–30 mg/dL. It must be stressed that these doses are
approximations and do not apply to patients in catabolic states (eg, sepsis, hyperthermia).
An alternative method is to administer regu-lar insulin as a continuous
infusion. The advantage of this technique is more precise control of insulin
delivery than can be achieved with a subcutaneous or intramuscular injection of
NPH insulin, partic-ularly in conditions associated with poor skin and muscle
perfusion. Regular insulin can be added to normal saline in a concentration of
1 unit/mL andthe infusion begun at 0.1 unit/kg/h. As blood glu-cose fluctuates,
the regular insulin infusion can be adjusted up or down as required. The dose
required may be approximated by the following formula:

A general target for the intraoperative mainte-nance of blood glucose is
less than 180 mg/dL. The tighter control afforded by a continuous intravenous
technique may be preferable in patients with type 1 diabetes.
When administering an intravenous insu-lin infusion to surgical
patients, adding some (eg, 20 mEq) KCl to each liter of fluid may be useful, as
insulin causes an intracellular potassium shift. Because individual insulin
needs can vary dramati-cally, any formula should be considered as only a crude
guideline.
If the patient is taking an oral hypoglycemic
agent preoperatively rather than insulin, the drug canbe
continued until the day of surgery. However, sulfonylureas and metformin have
long half-lives and many clinicians will discontinue
them 24–48 h before surgery. They can be started postop-eratively when the
patient resumes oral intake. Metformin is restarted if renal and hepatic
function remain adequate. The effects of oral hypoglycemic drugs with a short
duration of action can be prolonged in the presence of kidney failure. Many
patients main-tained on oral antidiabetic agents will require insulin treatment
during the intraoperative and postoperative periods. The stress of surgery
causes elevations in counterregulatory hormones (eg, catecholamines,
glucocorticoids, growth hormone) and inflammatory mediators such as tumor
necrosis factor and interleu-kins. Each of these contributes to stress
hyperglyce-mia, which increases insulin requirements. In general, type 2
diabetic patients tolerate minor, brief surgical procedures without any
exogenous insulin. However, many ostensibly “nondiabetic” patients show pro-nounced
hyperglycemia during critical illness and require a period of insulin therapy.
The key to any management regimen is to monitor plasma glucose levels
frequently. Patients receiving insulin infusions intraoperatively may need to
have their glucose measured hourly. Those with type 2 diabetes vary in their
ability to produce and respond to endogenous insulin, and measure-ment every 2
or 3 h may be sufficient. Likewise, insulin requirements vary with the
extensiveness of the surgical procedure. Bedside glucose meters are capable of
determining the glucose concen-tration in a drop of blood obtained from a
finger stick (or withdrawn from a central or arterial line) within a minute.
These devices measure the color conversion of a glucose oxidase–impregnated strip.
Their accuracy depends, to a large extent, on adherence to the device’s
specific testing proto-col. Monitoring urine glucose is of value only for
detecting glycosuria.
Patients who take NPH or other protamine-containing insulin preparations
have an increased risk of allergic reactions to protamine sulfate— including
anaphylactoid reactions and death. Unfortunately, operations that require the
use of heparin and subsequent reversal with protamine (eg, cardiopulmonary
bypass) are more common in diabetic patients. The usefulness of a small
prot-amine test dose of 1–5 mg over 5–10 min prior to the full reversal dose is
unclear, although this is recom-mended by some clinicians.
Patients who use subcutaneous insulin infu-sion pumps for management of
type 1 diabetes usually can leave the pump programmed to deliver “basal”
amounts of regular insulin (or insulin glargine). This is the amount of insulin
required during fasting. Such patients can safely undergo short outpatient
surgery with the pump on the basal setting. If more extensive inpatient
proce-dures are required, these patients will normally be managed with
intravenous insulin infusions as described earlier.
C. Postoperative
Close monitoring of blood glucose must continue postoperatively. There
is considerable patient-to-patient variation in onset and duration of action of
insulin preparations ( Table 34–5). For example, the onset of
action of subcutaneous regular insulin is less than 1 h, but in rare patients
its duration of action may continue for 6 h. NPH insulin typically has an onset
of action within 2 h, but the action can last longer than 24 h. Another reason
for close
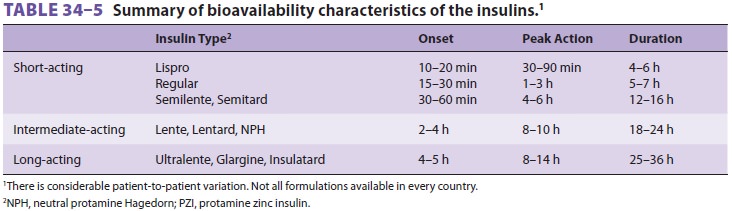
monitoring is the progression of stress hyperglyce-mia in the recovery
period.
Related Topics