Chapter: Clinical Anesthesiology: Anesthetic Management: Anesthesia for Trauma & Emergency Surgery
Anesthesia for Burns
BURNS
Burns represent a unique but common traumatic
injury that is second only to motor vehicle acci-dents as the leading source of
accidental death. Temperature and duration of heat contact determine the extent
of burn injury. Children (because of a high body surface area to body mass
ratio) and the elderly (whose thinner skin allows deeper burns from simi-lar
thermal insult) are at greater risk for major burn injury. The
pathophysiological and hemodynamic responses to burn injuries are unique and
warrant specialized burn care that can be optimally provided only at burn
treatment centers, particularly when more than 20% of a patient’s body surface
area is involved in second- or third-degree burns. A basic understanding of
burn pathophysiology and of resus-citation requirements, especially early
initiation of therapies such as oxygen administration and aggres-sive fluid resuscitation,
will improve patient survival.
Burns are classified as first, second, or
third degree. First-degree burns are
injuries that do not penetrate the epidermis (eg, sunburns and superficial
thermal injuries). Fluid replacement for these burns is not necessary, and the
area of first-degree burns should not be included in calculating fluid
replace-ment requirements when extensive, more significant burns are also
present. Second-degree burns are
par-tial-thickness injuries (superficial or deep) that pen-etrate the
epidermis, extend into the dermis for some depth, and are associated with
blistering. Fluid replacement therapy is indicated for patients with
second-degree burns when more than 20% of total body surface area (TBSA) is
involved. Skin grafting also may be necessary in some cases of second-degree
burns, depending upon size and location of the wounds. Third-degree burns are those in which the thermal injury penetrates
the full thickness of the dermis. Nerves, blood vessels, lymphatic channels, and
other deep structures may have been destroyed, creating a severe, but
insensate, wound (although surrounding tissue may be very painful). Debridement
and skin grafting are nearly always required for recovery of patients from
third-degree burns.
Major burns (a second- or third-degree burn involving
20% TBSA) induce a unique hemo-dynamic response. Cardiac output declines by up
to 50% within 30 minutes in response to massive vaso-constriction, inducing a
state of normovolemic hypoperfusion (burn shock). Survival depends on
restoration of circulating volume and infusion of crystalloid fluids according
to recommended proto-cols . This intense hemodynamic response may be poorly
tolerated by patients with significant underlying medical conditions. If
intra-venous fluid therapy is provided, cardiac function returns to normal
within 48 h of the injury, then typically progresses to a hyperdynamic
physiology as the metabolic challenge of healing
begins. Plasma volume and urine output are also reduced early on after
major burn injuries.In contrast to fl uid management for blunt and penetrating trauma, which
discourages use ofcrystalloid fluids, burn fluid resuscitation empha-sizes the use of
crystalloids, particularly lactated Ringer’s solution, in preference to
albumin, hydroxy-ethyl starch, hypertonic saline, and blood. Following burn
injuries, kidney failure is more common when hypertonic saline is used during
initial fluid resusci-tation, death is higher when blood is administered, and
outcomes are unchanged when albumin is used in resuscitation.
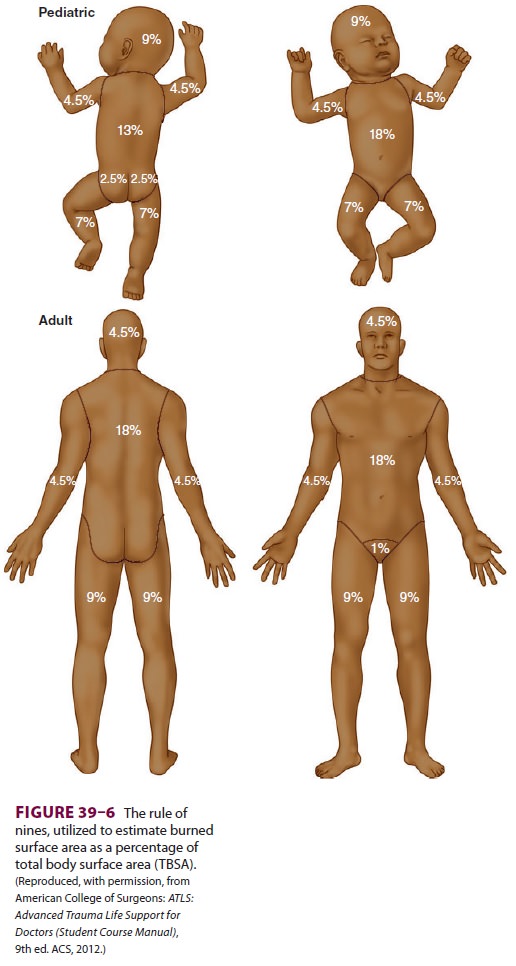
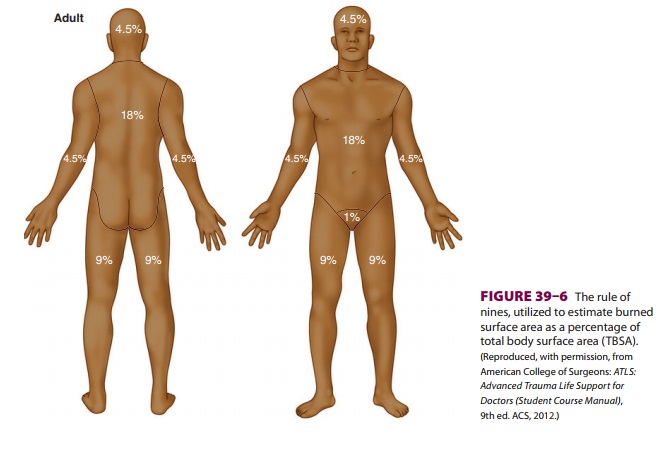
Fluid resuscitation is continuous over the
first 24 h following injury. Two formulas are commonly used to guide burn
injury fluid resuscitation, the Parkland and the modified Brooke. Both require
an understanding of the so-called rule of
nines (Figure 39–6) to calculate
resuscitation volumes. The (adult) Parkland
protocol recommends 4 mL/kg/% TBSA burned to be given in the first 24 h, with
half the volume given in the first 8 h and the remaining amount over the
following 16 h. The (adult) modi-fied
Brooke protocol recommends 2 mL/kg/% TBSA,with administration of half the
calculated volume beginning in the first 8 h and the remainder over the
following 16 h. Both formulas use urine output as a reliable indicator of fluid
resuscitation, target-ing (adult) urine production of 0.5–1.0 mL/kg/h as
indications of adequate circulating volume. If adult urine output exceeds 1.0
mL/kg/h, the infusion is slowed. In both protocols, an amount equal to half the
volume administered in the first 24 h is infused in the second 24-h period
following injury, with con-tinued attention to maintaining adult urine output
at 0.5–1.0 mL/kg/h. The formula for fluid resuscitation of children is the same
as that for adults, but children weighing less than 30 kg should receive 5%
dextrose in Ringer’s lactate as their resuscitation fluid and tar-get urine
output should be 1.0 mL/kg/h. The target urine output for infants younger than
1 year of age is 1–2 mL/kg/h.
Management Considerations
The Parkland and modified Brooke protocols both use urine output as an
indicator for adequate fluid resuscitation. However, circumstances may arise in
which the volume of fluid administered exceeds the intended volumes. For
example, initial fluid resusci-tation volumes may be miscalculated if
first-degree burns are mistakenly incorporated into the TBSA value. Prolonged
use of sedatives and sedative infu-sions may also result in hypotension that is
treated with additional fluids rather than vasoconstrictors. The phenomenon of fluid creep occurs when intra-venous
fluid therapy volumes are increased beyond intended calculations in response to
various hemo-dynamic changes. Fluid creep is associated with abdominal
compartment syndrome and pulmo-nary complications, which represent
resuscitation morbidity.
A. Abdominal Compartment Syndrome
Abdominal compartment syndrome is a risk for
pediatric patients, adults with circumferential abdominal burns, and patients
receiving intrave-nous fluid volumes greater than 6 mL/kg/% TBSA.
Intraabdominal pressure can be determined by measuring intraluminal bladder
pressure using a Foley catheter. The transducer is connected to a 3-way
stopcock at the point where the Foley cath-eter connects to the drainage tube.
After the trans-ducer is zeroed at the pelvic brim, 20 mL of fluid is instilled
to distend the bladder. Intraabdominal pressure readings are taken 60 s after
fluid installa-tion, allowing the bladder to relax. Intraabdominal pressures
exceeding 20 mmHg warrant abdominal cavity decompression. However, an abdominal
sur-gical procedure places the burn patient at high risk for intraabdominal Pseudomonas infection, particu-larly if
the laparotomy incision is near burned tissue.
B. Pulmonary Complications
Excessive resuscitative fluid volumes are associated with an increased incidence of pneumonia. Patients with severe burns frequently have pulmonary injury related to the burn. Decreased tracheal ciliary activ-ity, the presence of resuscitation-induced pulmo-nary edema, reduced immunocompetence, and tracheal intubation predispose the burn patient to pneumonia. Abdominal compartment syndrome can have an adverse impact on pulmonary function. Intravenous fluid administration volumes must be monitored closely and documented to be consistent with American Burn Association recommendations (ie, the Parkland or modified Brooke protocol).Fluid administration that exceeds recommenda-tions warrants careful review of the rationale for the increased fluid therapy volume, including assess-ment of possible causes for hypotension (eg, sepsis) or reduced urine output (eg, abdominal compart-ment syndrome).
C. Carbon Monoxide Poisoning
Carbon monoxide poisoning should be
con-sidered in all serious burn injury cases, as wellas with lesser TBSA burns
occurring in enclosed spaces. Unconsciousness or decreased levels of
con-sciousness following burn injuries should be pre-sumed to represent carbon
monoxide poisoning, prompting endotracheal intubation and mechanical
ventilation with high inspired concentration oxy-gen therapy. Carbon monoxide
binds to hemoglo-bin with an affinity approximately 250 times that of oxygen.
The resultant carboxyhemoglobin (HbCO) leaves less hemoglobin available to bind
with oxygen (HbO2) and shifts the O2–Hb dissociation curve to the left; both of these processes result in
impaired availability of oxygen molecules at the local tissue level. Pulse
oximetry provides a falsely elevated indication of oxygen saturation in the
setting of carbon monoxide exposure because of its inability to distinguish between
HbO2
and HbCO. If carbon monoxide poisoning is suspected, HbCO can be directly
measured via arterial or venous blood gas analysis. HbCO concentrations below
10% are usu-ally not clinically significant. However, with high inspired oxygen
concentrations, HbCO levels of 20% correspond to a hemoglobin oxygen
satura-tion of 80%; intubation and mechanical ventilation is indicated in such
circumstances to improve local tissue oxygenation and enhance carbon monoxide
elimination. Death from carbon monoxide poison-ing occurs at HbCO levels of
60%.
Anesthetic Considerations
A primary characteristic of all burn patients is an inability to
regulate temperature. The resuscitation environment must be maintained near
body tem-perature through the use of radiant warming, forced air warming
devices, and fluid warming devices.
Assessment of the patient begins with
inspec-tion of the airway. Although the face may be burned (singed facial hair,
nasal vibrissae), facial burns are not an indication for tracheal intubation.
The need for urgent airway management, mechanical venti-lation, and oxygen
therapy is indicated by hoarse voice, dyspnea, tachypnea, or altered level of
con-sciousness. Arterial blood gases should be obtained early in the treatment
process to assess HbCO levels.
Mechanical ventilation should be adjusted to
afford adequate oxygenation at the lowest tidal volumes.
Tracheal intubation in the early period follow-ing burn injury (up to
the first 48 h) can be facili-tated with succinylcholine for paralysis. In patients
with significant burns (>20% TBSA),
injuries and disruption of neuromuscular end plates occur fol-
lowed by upregulation of acetylcholine receptors.
Beyond 48 h after a major burn,
succinylcho-line administration is likely to produce potentially lethal elevation of serum potassium levels. Analgesia for burn
patients is challengingbecause of concerns about opioid tolerance and
psy-chosocial complications. Multimodal approaches are often advantageous.
Regional analgesia may provide benefit, although in the early postburn period
this technique may mask the symptoms of compartment syndrome or other clinical
signs and symptoms.
Related Topics