Chapter: Clinical Anesthesiology: Anesthetic Management: Anesthesia for Trauma & Emergency Surgery
Anesthesia for Resuscitation
RESUSCITATION
Hemorrhage
Certain trauma-related terminology must be understood and utilized in
order to effectively communicate with surgeons during trauma resus-citations or
surgeries in which blood loss is occur-ring. Hemorrhage classifications I–IV, damagecontrol resuscitation, and
damage control surgery are terms that quickly convey critical informa-tion
between surgeons and anesthesia personnel, ensuring a common understanding of
the various interventions that may be required to resuscitate a trauma or
surgical patient experiencing bleed-ing. The ACS identifies four classes of
hemorrhage. Understanding this classification scheme promotes more effective
communication between surgeons and anesthesiologists.
Class
I hemorrhage is the volume of blood thatcan be lost
without hemodynamic consequence. The heart rate does not change and the blood
pressure does not decrease in response to losing this volume of blood. In most
circumstances, this volume repre-sents less than 15% of circulating blood
volume. The typical adult has a blood volume equivalent to 70 mL/ kg. A 70-kg
adult can be presumed to have nearly 5 L of circulating blood. Children are
considered to have 80 mL/kg and infants, 90 mL/kg blood volume. Intravenous
fluid is not required if the bleeding is controlled, as in brief, controlled
bleeding encoun-tered during an elective surgical procedure.
Class
II hemorrhage is the volume of blood,that, when lost,
prompts sympathetic responses to maintain perfusion; this usually represents
15–30% of circulating blood volume. The diastolic blood pressure will increase
(a reflection of vasoconstric-tion) and the heart rate will increase to
maintain car-diac output. Intravenous fluid or colloid is usually indicated for
blood loss of this volume. Transfusions may be required if bleeding continues,
suggesting progression to class III hemorrhage.
Class III hemorrhage represents the volume of
blood loss (30–40% of circulating blood vol-ume) that consistently results in
decreased blood pressure. Compensatory mechanisms of vasocon-striction and
tachycardia are not sufficient to main-tain perfusion and meet the metabolic
demands of the body. Metabolic acidosis will be detected on arterial blood gas
analysis. Blood transfusions are necessary to restore tissue perfusion and
pro-vide oxygen to tissues. The patient may transiently respond to fluid
boluses given in response to hem-orrhage; however, if bleeding persists or
given time for the fluid bolus to redistribute, the blood pres-sure will
decline. Surgeons should be advised when this pattern persists, particularly
during elective surgical cases where the development of shock is not expected.
Class III hemorrhage may prompt an intervention such as a damage control
procedure .
Class
IV hemorrhage represents life-threaten-ing hemorrhage. When
more than 40% of circulating blood volume is lost, the patient will be
unrespon-sive and profoundly hypotensive. Rapid control of bleeding and
aggressive blood-based resuscitation (ie, damage control resuscitation) will be
required to prevent death. Patients experiencing this degree of hemorrhage will
likely develop a trauma-induced coagulopathy, require massive blood
transfusion, and experience a high likelihood of death.
Trauma-Induced Coagulopathy
Coagulation abnormalities are common
following major trauma, and trauma-induced coagulopathy is an independent risk factor for death. Recent
prospective clinical studies suggest that in upto 25% of major trauma patients,
trauma-induced coagulopathy is present shortly after injury and before any
resuscitative efforts have been initiated. In one report, acute traumatic
coagulopathy was only related to the presence of a severe metabolic acidosis
(base deficit ≥6 mEq/L) and appeared to have a dose-dependent relationship with
the degree of tissue hypoperfusion; 2% of patients with base deficits less than
6 mEq/L developed coagulopathy compared with 20% of patients with base deficits
greater than 6 mEq/L. Although injury severity scores were likely high in those
developing coagulopathy, only the
presence of the metabolic acidosis correlated
to developing trauma-induced coagulopathy. Global tissue hypoperfusion appears
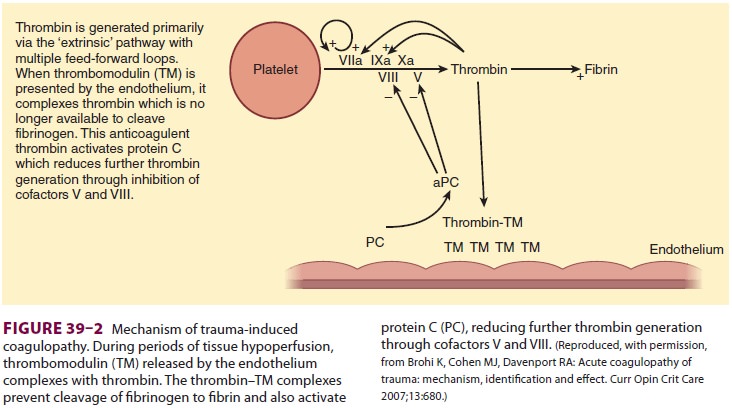
to have a key role in the development of trauma-induced coagulopathy. During
hypoperfusion, the endo-thelium releases thrombomodulin and activated protein C
to prevent microcirculation thrombosis. Thrombomodulin binds thrombin, thereby
prevent-ing thrombin from cleaving fibrinogen to fibrin. The
thrombomodulin–thrombin complex activates protein C, which then inhibits the
extrinsic coagu-lation pathway through effects on cofactors V and VIII (Figure
39–2). Activated protein C also inhibits
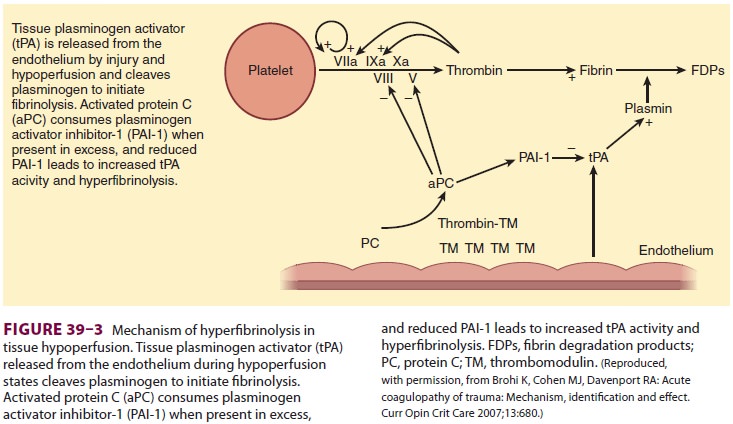
plasminogen activator inhibitor-1 proteins, which increases tissue plasminogen
activator, resulting in hyperfibrinolysis (Figure 39–3). One prospective clinical study found the following effects of
hypoper-fusion on coagulation parameters: (1) progressive coagulopathy as base
deficit increases; (2) increas-ing plasma thrombomodulin and falling protein C
(indicating activation of the protein levels with increasing base deficit),
supporting the argument that the anticoagulant effects of these proteins in the
presence of hypoperfusion are related to the prolon-gation of prothrombin and
partial thromboplastin times; and (3) an influence of early trauma-induced
coagulopathy on mortality.
Trauma-induced coagulopathy is not solely
related to impaired clot formation. Fibrinolysis is an equally important
component as a result of plas-min activity on an existing clot. Tranexamic acid
administration is associated with decreased bleed-ing during cardiac and
orthopedic surgeries, pre-sumably because of its antifibrinolytic properties. A
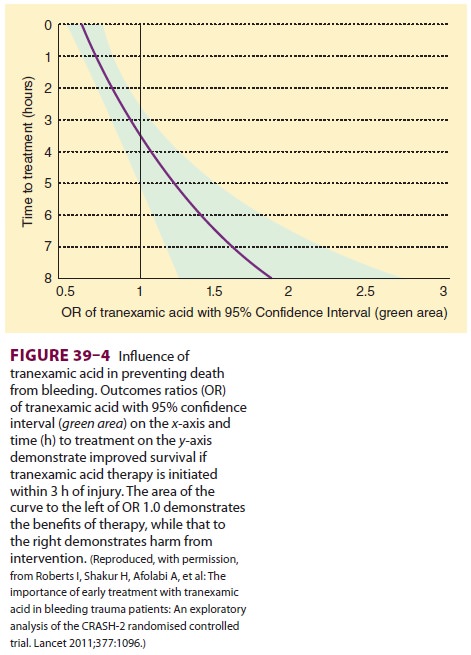
randomized control study involving 20,000 trauma patients with or at risk of
significant bleeding found a significantly reduced risk for death from
hemor-rhage when tranexamic acid therapy (loading dose, 1 g over 10 min
followed by an infusion of 1 g over 8 h) was initiated within the first 3 h
following major trauma. Figure 39–4
demonstrates the benefit of ini-tiating this therapy in relation to the time of
injury.
Hemostatic Resuscitation
Early coagulopathy of trauma is associated with increased mortality. Administering blood products in equal ratios early in resuscitationhas become an accepted approach to correction of trauma-induced coagulopathy. This balanced approach to transfusion, 1:1:1 (red blood cell:fresh frozen plasma:platelet), is termed damage controlresuscitation. Although the 1:1:1 combinationattempts to replicate whole blood, it results in a pan-cytopenic solution with only a fraction of whole blood’s hematocrit and coagulation factor concen-tration. Red blood cells will over time improveoxygen delivery to ischemic, hypoperfused tissues. Fresh frozen plasma provides clotting factors V and VIII along with fibrinogen, which improves clotting, possibly due to overwhelming of the thrombin– thrombomodulin complex. Platelets and cryopre-cipitate, although included in the 1:1:1 massive transfusion protocol, are probably not necessary in the initial phase of resuscitation, given the normal platelet and fibrinogen levels noted in early coagu-lopathy. Additional platelet transfusions may be beneficial if the resuscitation is prolonged, as is typi-cal for most major trauma resuscitations, or if a recalcitrant coagulopathy is noted with coagulation studies. The use of crystalloid fluids in early trauma resuscitation has markedly decreased with the increased emphasis upon early blood product administration.
Most trauma centers have early-release type
O-negative blood available for immediate transfu-sion to patients with severe
hemorrhage. Depending on the urgency of need for transfusion, administra-tion
of blood products typically progresses from O-negative to type-specific, then
to crossmatched units as the acute need decreases. Patients admin-istered uncrossmatched
O-negative blood are those deemed at high risk of requiring massive
trans-fusion. As the amount of uncrossmatched blood administered increases
beyond 8 units, attempts to return to the patient’s native blood type should
not be pursued and type O blood should be continued until the patient is
stabilized.
Military experience treating combat-wounded
soldiers and civilians has provided great insight into trauma resuscitation and
trauma-induced coagu-lopathy. As the use of blood and blood products has
evolved, the 1:1:1 transfusion ratio has been uni-formly adopted to address the
frequent incidence of trauma-induced coagulopathy. Retrospective analy-sis of
severely wounded solders found improved survival when this transfusion protocol
was utilized. Consequently, hemostatic resuscitation has been rapidly adopted
by civilian trauma centers, which have reported similar survival benefits for
civilian patients with severe trauma. Nevertheless, using tra-ditional
definitions, this approach is not “evidence based” from randomized clinical
trials.
Using hemostatic resuscitation (ie, damage control resuscitation), blood
and blood products are administered preemptively to address a pre-sumed
coagulopathy. Often coagulation status is not assessed until the patient
stabilizes. Although this treatment approach appears to be effective in
controlling trauma-induced coagulopathy, patients requiring this therapy may be
exposed to unneces-sary additional units of blood or blood products. An
alternative approach that relies on thromboelastog-raphy (TEG) may allow more
goal-directed transfu-sion of blood and blood products and is increasingly
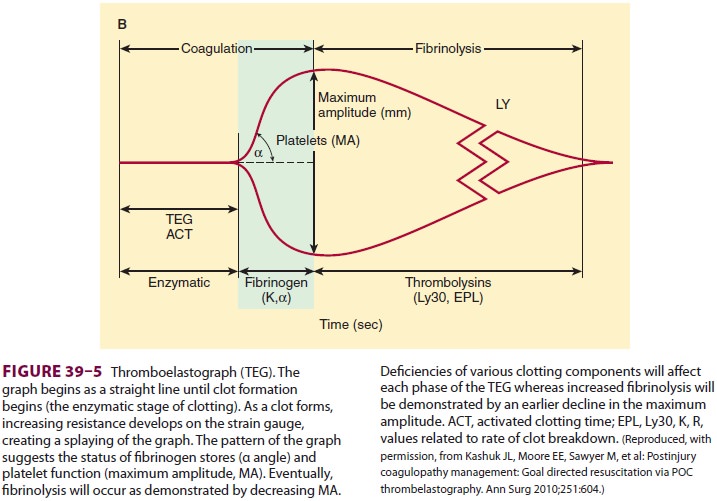
utilized in trauma resuscitations. The formation and stability of a clot
represents interactions between the coagulation cascades, platelets, and the
fibrinolytic system, all of which can be demonstrated with TEG (Figure
39–5). As TEG use during trauma resus-citation becomes more routine, the
current 1:1:1 hemostatic resuscitation ratio will likely undergo modification
to proportionately less fresh frozen plasma, and the use of antifibrinolytic
therapy will likely increase.
Administration of blood products must be done
with consideration for potential hazards that may result from transfusion.
Although blood-borne dis-eases such as acquired immunodeficiency syndrome,
hepatitis B, and hepatitis C are usually thought of as the highest
transfusion-related risks, the incidence of such infections has decreased
10,000-fold due to better screening tests of donors and donated units. Noninfectious
transfusion reactions are now the leading complication oftransfusion and
represent a more than 10-fold greater risk than blood-borne infection.
Transfusion-related acute lung injury (TRALI) is the leading cause of
transfusion-related death reported to the U.S. Food and Drug Administration.
However, although the bleeding trauma patient is at risk for a
transfusion-related reaction, that risk is minimal compared with the far
greater likelihood of death from exsanguination. The most prudent approach for blood
product utilization in the bleeding trauma patient is to administer the blood
products that are necessary, based on laboratory studies, clinical evi-dence of
significant bleeding, and the degree of hemodynamic instability that can be
directly attrib-uted to hemorrhage.
Massive Transfusion Protocols
Delay in obtaining blood products other than red blood cells is common in both civilian and military settings. Clinical evidence supports the need for, and benefit of, established massive transfusion protocols (MTPs), allowing the blood bank to assemble blood products in prescribed ratios to support hemostatic resuscitation. With MTPs in place, hemostatic resus-citation can continue until the demand for blood products stops. An MTP-driven, blood-based resus-citation, rather than a crystalloid-based resuscita-tion, improves survival from trauma, reduces total blood product utilization in the first 24 h follow-ing injury, reduces acute infectious complications (severe sepsis, septic shock, and ventilator-associated pneumonia), and decreases postresuscitation organ dysfunction (an 80% decrease in odds of developing multisystem organ failure).
It is important to establish which personnel are empowered to invoke use
of the MTP, given the expense and implications for the blood bank in terms of
blood inventory, personnel training and availability, and disruption of routine
blood bank duties. Establishing an MTP benefits both the patient, through
improved survival and fewer complications, and the institution, through more
efficient and effective processes for utilizing critical blood bank resources.
Initiating an MTP for all trauma patients is impractical; however,
delaying request for an MTP until the patient has undergone
a thorough trauma evaluation may increase the risk of morbidity and mortality.
The assessment of blood consump-tion (ABC) score is an attempt to predictwhich
patients are likely to require an MTP. The ABC score assigns 1 point for the
presence of each of four possible variables: (1) penetrating injury; systolic blood pressure less than 90 mmHg; (3) heart rate greater than
120 beats per minute; and
positive results of a focused
assessment withsonography for trauma (FAST) evaluation. TheFAST evaluation
is a bedside ultrasonography screening examination performed by surgeons and
emergency department physicians to assess the presence or absence of free fluid
in the perihepatic and perisplenic spaces, pericardium, and pelvis.Patients
with ABC scores of 2 or higher are likely to require massive transfusion. This
scoring system has been validated in multiple level 1 trauma cen-ters and is
now relatively commonplace in trauma evaluations.
Related Topics