Chapter: Clinical Anesthesiology: Anesthetic Management: Anesthesia for Patients with Respiratory Disease
Anesthesia : Pulmonary Embolism
Pulmonary Embolism
Preoperative Considerations
Pulmonary embolism results from the
entry of blood clots, fat, tumor cells, air, amniotic fluid, or foreign
material into the venous system. Clots from the lower extremities, pelvic
veins, or, less com-monly, the right side of the heart are usually
respon-sible. Venous stasis or hypercoagulability is often contributory in such
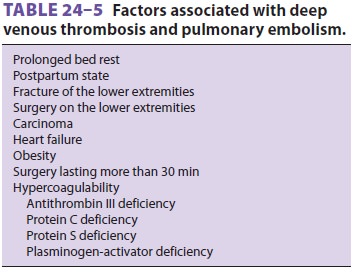
cases (Table24–5).
Pulmonary embolism can also occur intraoperatively in normal individuals
undergoing certain procedures.
A. Pathophysiology
Embolic occlusions in the pulmonary
circula-tion increase dead space, and, if minute ventilation does not change,
this increase in dead space should theoretically increase Paco2. However, in practice, hypoxemia is more often
seen. Pulmonary emboli acutely increase pulmonary vascular resistance by
reducing the cross-sectional area of the pulmonary vasculature,causing reflex
and humoral vasocon-striction. Localized or generalized reflex
bronchoconstriction further increases areas with low (V/Q)ratios. The net effect is an increase in
V/Q mismatch and hypoxemia. The affected area loses its
surfactant within hours and may become atelectatic within 24–48 hr. Pulmonary
infarction occurs if the embolus involves a large vessel and collateral blood
flow from the bronchial circulation is insufficient for that part of the lung
(incidence <10%). In previously healthy persons,
occlusion of more than 50% of the pulmo-nary circulation (massive pulmonary
embolism) is necessary before sustained pulmonary hypertension is seen.
Patients with preexisting cardiac or pulmo-nary disease can develop acute
pulmonary hyperten-sion with occlusions of lesser magnitude. A sustained
increase in right ventricular afterload can precipitate acute right ventricular
failure. If the patient survives acute pulmonary thromboembolism, the thrombus
usually begins to resolve within 1–2 weeks.
B. Diagnosis
Clinical manifestations of pulmonary
embolism include sudden tachypnea, dyspnea, chest pain, or hemoptysis. The
latter generally implies lung infarction. Symptoms are often absent or mild and
nonspecific unless massive embolism has occurred. Wheezing may be present on
auscultation. Arterial blood gas analysis typically shows mild hypox-emia with
respiratory alkalosis (the latter due to an increase in ventilation). The chest
radiograph is commonly normal, but may show an area of oli-gemia (radiolucency),
a wedge-shaped density with an infarct, atelectasis with an elevated diaphragm,
or an asymmetrically enlarged proximal pulmo-nary artery with acute pulmonary
hypertension. Cardiac signs include tachycardia and wide fixed splitting of the
S2 heart sound; hypotension with elevated
central venous pressure is usually indica-tive of right ventricular failure.
The electrocardio-gram frequently shows tachycardia and may show signs of acute
cor pulmonale, such as new right axis deviation, right bundle branch block, and
tall peaked T waves. Ultrasound studies of the lower extremities also may be
helpful in demonstrating deep venous thrombosis. The diagnosis of embo-lism is
more difficult to make intraoperatively .
Pulmonary angiography is still the gold
stan-dard criterion for diagnosing a pulmonary embo-lism, but it is invasive
and difficult to perform. Therefore, the less invasive spiral computed
tomog-raphy angiography (CTA) is the initial imaging of choice in stable
patients with suspected pulmonary embolism. Ventilation-perfusion (V/Q
) scanning may also be used when CTA cannot be performed. High-, intermediate-,
and low-probability criteria have been established for the diagnosis of
pulmonary embolism byV/Q scan.
C. Treatment and Prevention
The best treatment for pulmonary
embolism is pre-vention. Heparin (unfractionated heparin 5000 U subcutaneously
every 12 h begun preoperatively or immediately postoperatively in high-risk
patients), enoxaparin or other related compounds, oral anti-coagulation
(warfarin), aspirin, or dextran therapy, together with early ambulation, can
all be used to reduce the incidence of deep vein thrombosis. The use of high
elastic stockings and pneumatic com-pression of the legs may also decrease the
incidenceof venous thrombosis in the legs, but not in the pel-vis or the
heart.After a pulmonary embolism, systemic anti-coagulation prevents the
formation of new blood clots or the extension of existing clots. Heparin
therapy is begun with the goal of achieving an acti-vated partial thromboplastin
time of 1.5–2.4 times normal. Low molecular-weight heparin (LMWH) is as
effective and is given subcutaneously at a fixed dose (based on body weight)
without labo-ratory monitoring. In high-risk patients, LMWH is started either
12 hr before surgery, 12–24 hr after surgery, or at 50% the usual dose 4–6 hr
after surgery. All patients should start warfarin therapy concurrent with
starting heparin therapy, and the two should overlap for 4–5 days. The
international normalized ratio should be within the therapeu-tic range on two
consecutive measurements, at least 24 hr apart, before the heparin is stopped.
Warfarin should be continued for 3–12 months. Thrombolytic therapy with tissue
plasminogen activator or streptokinase is indicated in patients with massive
pulmonary embolism or circula-tory collapse. Recent surgery and active
bleed-ing are contraindications to anticoagulation and thrombolytic therapy. In
these cases, an inferior vena cava umbrella filter may be placed to prevent
recurrent pulmonary emboli. Pulmonary embo-lectomy may be indicated for
patients with mas-sive embolism in whom thrombolytic therapy is
contraindicated.
Anesthetic Considerations
A. Preoperative Management
Patients with acute pulmonary embolism
may pres-ent in the operating room for placement of an IVC filter, or, rarely,
for pulmonary embolectomy. In most instances, the patient will have a history
of pulmonary embolism and presents for unrelated surgery; in this group of
patients, the risk of inter-rupting anticoagulant therapy perioperatively is
unknown. If the acute episode is more than 1 year old, the risk of temporarily
stopping anticoagulant therapy is probably small. Moreover, except in the case
of chronic recurrent pulmonary emboli, pul-monary function has usually returned
to normal. The emphasis in the perioperative management of these patients
should be in preventing new episodes of embolism (see above).
B. Intraoperative Management
Vena cava filters are usually placed
percutaneously under local anesthesia with sedation.
Patients presenting for pulmonary
embo-lectomy are critically ill. They are usually already intubated, but
tolerate positive-pressure ventila-tion poorly. Inotropic support is necessary
until the clot is removed. They also tolerate all anesthetic agents very poorly.
Small doses of an opioid, etomi-date, or ketamine may be used, but the latter
can theoretically increase pulmonary artery pressures. Cardiopulmonary bypass
is required.
C. Intraoperative Pulmonary Embolism
Significant pulmonary embolism is a rare
occurrence during anesthesia. Diagnosis requires a high index of suspicion. Air
emboli are common, but are often overlooked unless large amounts are entrained.
Fat embolism can occur during orthopedic procedures; amniotic fluid embolism is
a rare, unpredictable, and often fatal, complication of obstetrical delivery.
Thromboembolism may occur intraoperatively dur-ing prolonged procedures. The
clot may have been present prior to surgery or may form intraoperatively;
surgical manipulations or a change in the patient’s position may then dislodge
the venous thrombus. Manipulation of tumors with intravascular extension can
similarly produce pulmonary embolism.
Intraoperative pulmonary embolism
usu-ally presents as sudden cardiovascular collapse, hypoxemia, or bronchospasm.
A decrease in end-tidal CO2 concentration is also suggestive of
pulmonary embolism, but is not specific. Invasive monitoring may reveal
elevated central venous and pulmonary arterial pressures. Depending on the type
and location of an embolism, a transesopha-geal echocardiogram may be helpful.
TEE may not reveal the embolus but will often demonstrate right heart
distention and dysfunction. If air is identified in the right atrium, or if it
is suspected, emergent central vein cannulation and aspiration of the air may
be lifesaving. For all other emboli, treatment is supportive, with intravenous
fluids and inotropes. Placement of a vena cava filter should be considered
postoperatively.
Related Topics