Chapter: Medicine Study Notes : Endocrine and Electrolytes
Acid-Base balance - Electrolytes
Acid-Base balance
Physiology
·
Metabolism produces two acids:
o Volatile: carbonic
o Non-volatile: eg lactic
·
Buffer systems:
o H+ + HCO3- « H2CO3 (H2O
+ CO2)
o Henderson Hasselbach Equation:

o Normal range for pH is 7.35 – 7.45 (=45 – 35 nmol/L of H+ ion)
o Range of pH compatible with life is about 6.8 – 7.8 = H+ concentration
of 160 – 16 nmol/l
o Lots of other buffering systems
·
Compensation:
o Never complete
o Respiratory: pH measured in the medulla.
Compensates rapidly
o Renal:
§ Alter bicarbonate reabsorption
§ Titratable acid excretion: organic buffers in tubules acidifies urine.
Excretes 30 – 50% of acid produced each day
§ NH4 excretion: formed in tubules,  takes days. Excretes 50 – 70% of acid
Respiratory Alkalosis
·
Hyperventilation
·
Causes:
o Hypoxia
o Lung disease: PE, asthma
o Anxiety
o Fever, sepsis
o Salicylate overdose: stimulates respiration, will subsequently develop
metabolic acidosis
·
ÂŻPaCO2, ÂpH, initial alterations in [HCO3] are minimal, if it persists
then kidneys compensate
·
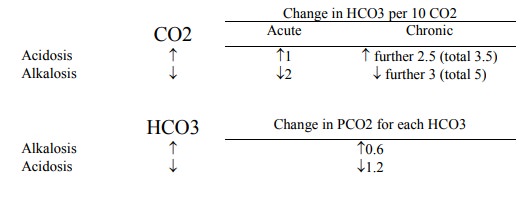
Compensation:
o Acute: HCO3 ÂŻ by 2 for each 10 ÂŻ PCO2
o Chronic: HCO3 ÂŻ by a further 3 (ie total of 5)
for each 10 ÂŻ PCO2 [renal loss of HCO3]
Respiratory Acidosis
·
Hypoventilation
·
Causes:
o PCO2 excretion lags production – eg severe asthma (initially
asthmatics hyperventilate)
o Pulmonary disease, muscular diseases, etc
o CNS depression: primary or drugs/toxins
o Asphyxia, smoke inhalation
·
As PCO2Â then CO2
+ H20 ® H+ + HCO3-
·
ÂPaCO2 ® ÂŻpH, initial alterations in [HCO3] are minimal, if it persists
then kidneys compensate (ÂHCO3 reabsorption, ÂNH3 formation and excretion):
o Acute: HCO3Â by 1 for each 10 ÂPCO2
o Chronic: HCO3Â by a further 2.5 (ie 3.5 of total) for each 10 ÂPCO2
o For example:
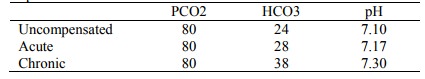
Metabolic acidosis
·
Net gain of acid
·
Causes:
o Accumulation of acid (anion gap > 18 mmol/L): ÂH+
(ketoacidosis, lactic acidosis, ingestion of salicylates, methanol), renal
failure (failure to excrete H+)
o ÂŻHCO3
(anion gap < 18 mmol): GI tract loss (eg diarrhoea), renal loss (eg ÂŻcarbonic
anhydrase), hypoaldosteronism
·
Compensation:
o Rapid: PCO2ÂŻ by 1.2 for each ÂŻ1 in HCO3 (baseline = 24) - rapid
o Slow: ÂHCO3 reabsorption and ÂNH4 excretion by the kidneys
Metabolic alkalosis
·
Net loss of acid
·
Causes:
o Loss of H+:
§ Vomiting (suspect surreptitious if low Cl)
§ NG suction
§ Renal loss (hyperaldosteronism)
o Increase in HCO3 reabsorption:
§ K depletion (Conn‟s, Cushing‟s, drugs, diuretics).
§ Volume depletion, eg ÂAldosteronism ® ÂNa/H exchange
o Gain in alkali: eg NaHCO3 administration
·
Compensation:
o PCO2 Â by 0.6 for each 1 ÂŻ in HCO3. Limited by
hypoxia
o Final compensation is by renal excretion of HCO3 – requires
correction of Cl, K and volume
Summary of compensation rules
Mixed Acid/Base disorders
·
Suspect if:
o Clinical grounds
o Compensation rules not obeyed
o Normal pH but abnormal PCO2 and HCO3
·
Examples:
o Respiratory + Metabolic Acidosis:
Pulmonary oedema + cardiac arrest
o Respiratory + Metabolic Alkalosis: Over-ventilation + Nasogastric
suction
o Respiratory Alkalosis + Metabolic Acidosis: Septic shock or Salicylate
OD
o Respiratory Acidosis + Metabolic Alkalosis: CORD + Diuretic
o Metabolic Acidosis + Metabolic Alkalosis: Renal failure + vomiting
Interpreting Blood Gas Results
·
Arterial blood taken in 2 ml
syringe containing heparin (to stop clotting) and transported on ice
·
Look at pH: 7.36 to 7.44 is
normal
· Look at PCO2. If < 36 then hyperventilation. If > 44 then hypoventilation.
·
Look at HCO3. If <
22 then metabolic acidosis. If > 26 then metabolic alkalosis. But HCO3
depends on PCO2. So (to work out if its just compensation, or there
is a metabolic problem as well as a respiratory one):
o For acute changes (hours): a fall in PaCO2 ® a normal
HCO3 2 less for every 10 mmHg ÂŻ in PaCO2. A rise in
PaCO2 ® normal HCO3 1 greater for every 10 mm Hg  in PaCO2
o For chronic changes (days): a rise in PaCO2 results in a
normal HCO3 4 greater for every 10 change in PaCO2
Base Excess
·
Given on all arterial blood gas
results
·
= Concentration of titratable
base when titrating blood or plasma with a strong acid or base to a plasma pH
of 7.40 at PCO2 of 40 mmHg at 37C
·
Intent is to remove the impact of
the respiratory component leaving just the metabolic component:
o If +ive: metabolic alkalosis ® deficit of non-carbonic acid
o If –ive: metabolic acidosis ® excess of non-carbonic acid
· BUT recognises normal compensation as an extra disturbance. May be useful for an anaesthetist (eg simple and acute disturbances
Related Topics