Chapter: Genetics and Molecular Biology: Regulation of Mating Type in Yeast
α2 and MCM1
α2 and MCM1
DNAse footprinting revealed an unexpectedly large
binding site for α2. It had two protected regions
around a central unprotected region. Since the central protected region
possessed a symmetric sequence, it seemed possible that an additional protein
bound there.
Two kinds of experiments could be used to look for
a possible second protein. The first was to ask whether the central region of
the α2-binding site is involved in repression, and the
second was to dissect α2 protein
into functional domains. It is relatively straightforward to construct and
insert altered α2-binding sites in front of
genes. When this was done, it was found that if their sites were altered in the
central region, repression did not occur despite the binding of α2. This means that either the central region
affects the activity of the bound protein without affecting its binding, or
that an additional protein binds in this region by making use of the central
region DNA sequence.
The DNA migration retardation assay was used to
look for a protein that binds with α2 to
repress genes. One was found. It could be purified, and its binding to the site
studied. It binds cooperatively with α2
protein.
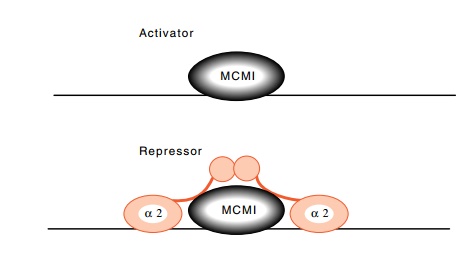
Figure
16.12 The MCM1 protein functions as an
activator when alone, and asa repressor when it is flanked by the α2 protein.
The sequence to which it binds is found in front of
some other genes whose activity is regulated by the mating type of the cells.
In some cases the protein is an activator, and in others it is a repressor.
When the α2 sequences flank the site, the
complex acts as a repressor (Fig. 16.12). This protein, which has finally come
to be called MCM1 has also been found in other types of cells. Its close
relative is found in human cells where it is a transcription factor that
activates genes in response to the presence of serum and is known as the serum
response factor.
The α2 protein
could also be dissected by deleting DNA segments from a plasmid containing the α2-β-galactosidase
fusion gene. The resulting proteins lost their DNA-binding activities only when
a 60 amino acid portion near the carboxyl terminus of the protein was deleted.
These proteins lost their ability to repress, however, when short stretches of
amino acids near the N-terminus were deleted. The resulting proteins could
still bind to DNA. Thus they were synthesized, stable, capable of binding to
DNA, but had lost a domain required for repres-sion.
Related Topics