Chapter: Plant Biochemistry: Mitochondria are the power station of the cell
The mitochondrial respiratory chain shares common features with the photosynthetic electron transport chain
The mitochondrial respiratory chain shares common features with the photosynthetic electron transport chain
The photosynthesis of cyanobacteria led to the accumulation of oxygen in the early atmosphere, which was the basis for the oxidative metabolism of mitochondria. Many cyanobacteria can satisfy their ATP demand both by photosynthesis and by oxidative metabolism. Cyanobacteria contain a photo-synthetic electron transport chain that consists of three modules (complexes), namely, photosystem II, the cyt-b6/f complex, and photosystem I. These complexes are located in the inner membrane of cyano-bacteria, where the enzymes of the respiratory electron transport chain are also localized. This respiratory chain consists of three modules: an NADH dehydrogenase complex, catalyzing the oxidation of NADH; the same cyt-b6/f complex that is also part of the photosynthetic electron transport chain; and a cyt-a/a3 complex, by which oxygen is reduced to water. Plastoquinone feeds the electrons into the cyt-b6/f complex not only in photosynthesis (sec-tion 3.7), but also in the respiratory chain of the cyanobacteria. Likewise, cytochrome-c mediates the electron transport from the cyt-b6/f complex to photosystem I as well as to the cyt-a/a3 complex. The relationship between photosynthetic and oxidative electron transport in cyanobacteria is obvious; both electron transport chains possess the same module as the middle of the reaction sequence, the cyt-b6/f complex. The function of the cyt-b6/f complex in respiration and photosynthesis shows that the principle of energy conservation in photosynthetic and oxidative electron transport is the same.
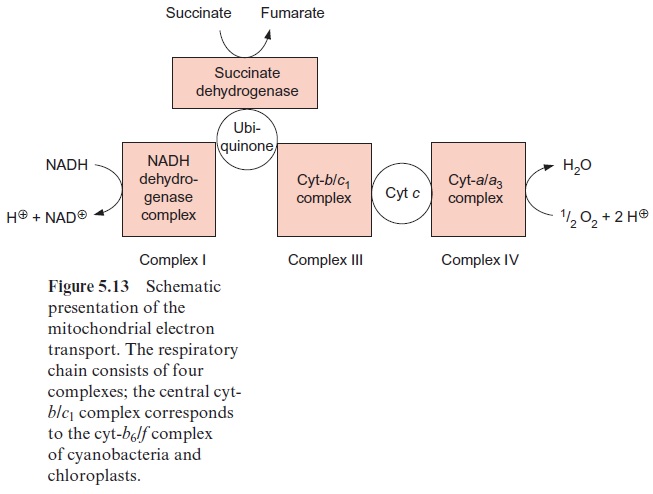
The mitochondrial respiratory chain is analogous to the respiratory chain of cyanobacteria (Fig. 5.13), but with ubiquinone instead of plasto-quinone as redox carrier and slightly different cytochromes. The mitochon-dria contain a related cyt-b/c1 complex instead of a cyt-b6/f complex, but both cyt-c and cyt-f contain heme-c.
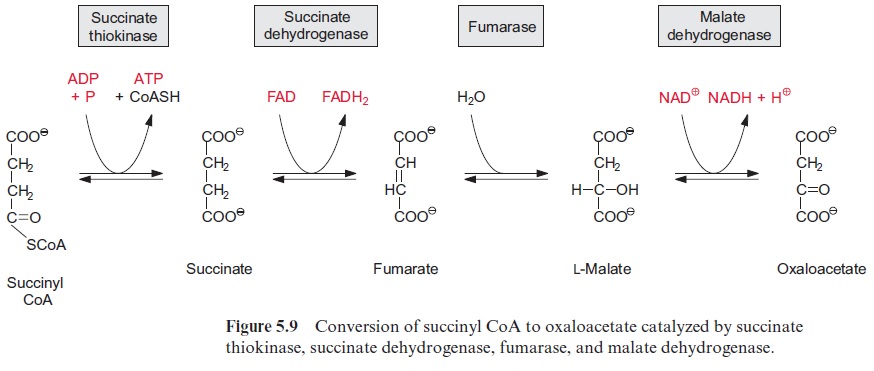
Figure 5.13 shows succinate dehydrogenase, another electron acceptor of the mitochondrial respiratory chain. This enzyme (historically termed complex II) catalyzes the oxidation of succinate to fumarate, a step of the citrate cycle (Fig. 5.9).

The complexes of the mitochondrial respiratory chain
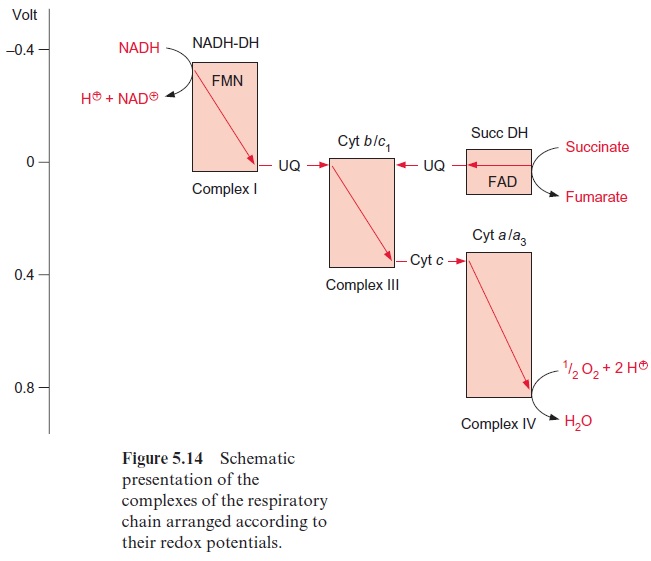
The subdivision of the respiratory chain into several complexes goes back to the work of Youssef Hatefi (USA, 1962), who succeeded in isolating four different complexes, which he termed complexes I–IV, while working with beef heart mitochondria. In the complexes I, III, and IV, the electron trans-port is accompanied by a decrease in the redox potential (Fig. 5.14); the energy thus released creates a proton gradient.
The NADH dehydrogenase complex (complex I) (Fig. 5.15) feeds the res-piratory chain with the electrons from NADH formed from the degrada-tion of substrates in the matrix. The electrons are transferred to ubiquinone via a flavin adenine mononucleotide (FMN) and several iron-sulfur centers.
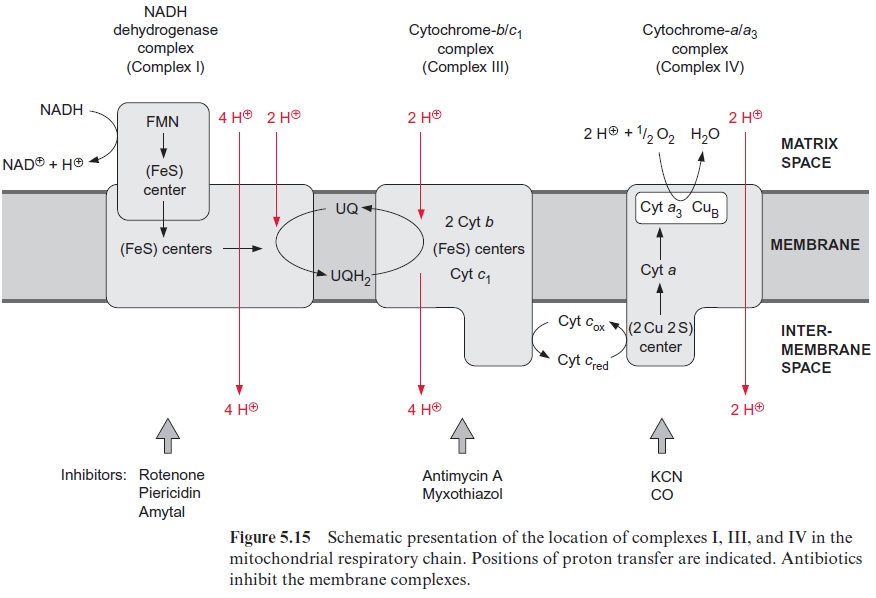
Complex I has the most complicated structure of all the mitochondrial elec-tron transport complexes. It consists of more than 40 different subunits (of which, depending on the organism, seven to nine are encoded in the mito-chondria). Part of the complex is embedded in the membrane (membrane part) and a peripheral part protrudes into the matrix space. The peripheral part provides the binding site for NADH and comprises FMN (Fig. 5.16) and at least three Fe-S-centers (Fig. 3.26). The membrane part contains another Fe-S-center, as well as the binding site for ubiquinone. The elec-tron transport can be inhibited by a variety of poisons deriving from plants and bacteria, such as rotenone (which protects plants from being eaten by animals); the antibiotic piericidin A; and amytal, a barbiturate. The elec-tron transport catalyzed by complex I is reversible. It is therefore possible for electrons to be transferred from ubiquinone to NAD+ , driven by the proton motive force of the proton gradient. In this way the NADH dehy-drogenase complex can provide purple bacteria with NADH (see Fig. 3.1).
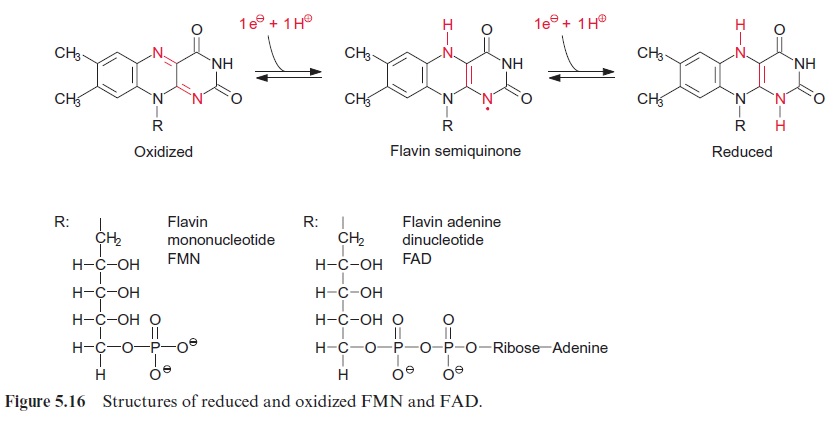
In plants the succinate dehydrogenase (complex II) (Fig. 5.9) consists of seven subunits comprising a flavin adenine nucleotide (FAD, Fig. 5.16) as the electron acceptor; several Fe-S-centers (Fig. 3.26) as redox carriers; and one cytochrome-b, of which the function is not known. Electron transport by succinate dehydrogenase to ubiquinone proceeds with no major decrease in the redox potential, so no energy is gained in the electron transport from succinate to ubiquinone.
Ubiquinone reduced by the NADH dehydrogenase complex or succi-nate dehydrogenase is oxidized by the cyt-b/c1 complex (complex III) (Fig. 5.15). In mitochondria this complex consists of 11 subunits, only one of which (the cyt-b subunit) is encoded in the mitochondria. The cyt-b/c1 com-plex is very similar in structure and function to the cyt-b6/f complex of chlo-roplasts. Electrons are transferred by the cyt-b/c1 complex to cyt-c, which is bound to the outer surface of the inner membrane. Several antibiotics, such as antimycin A and myxothiazol, inhibit the electron trans-port by the cyt-b/c1 complex.
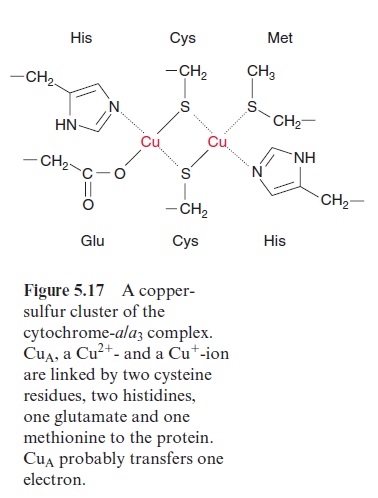
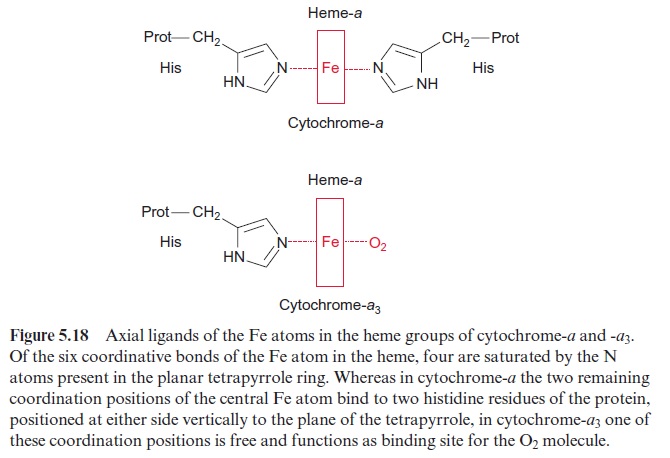
Due to its positive charge, reduced cyt-c diffuses along the negatively charged surface of the inner membrane to the cyt-a/a3 complex (Fig. 5.15), also termed complex IVor cytochrome oxidase. The cyt-a/a3 complex con-tains 13 different subunits, three of which are encoded in the mitochon-dria. The three-dimensional structures of the beef heart mitochondrial and Paracoccus denitrificans cyt-a/a3 complex have been resolved by X-ray crys-tallography. The complex has a large hydrophilic region that protrudes into the intermembrane space and provides the binding site for cyt-c. During the oxidation of cyt-c the electrons are transferred to a copper sulfur clustercontaining two Cu atoms called CuA. These two Cu atoms are linked by two S-atoms of cysteine side chains (Fig. 5.17). This copper-sulfur cluster probably takes up one electron and transfers it via cyt-a to a binuclear center, consisting of cyt-a3 and a Cu atom (CuB), bound to histidine. This binuclear center functions as a redox unit in which the Fe atom of the cyt-a3, together with CuB, take up two electrons.
[Fe+++ . CuB++] + 2e- → [Fe++ . CuB+]
In contrast to cyt-a and the other cytochromes of the respiratory chain, the sixth coordination position of cyt-a3 of the heme Fe atom is not satu-rated by an amino acid of the protein (Fig. 5.18). This free coordination position as well as CuB are the binding site for the oxygen molecule, which is reduced to water by the uptake of four electrons:
O2 + 4 e- → 2 O2- + 4 H+ → 2 H2O
Furthermore, CuB probably has an important function in electron-driven proton transport. Instead of O2, also CO and CN- can be very tightly bound to the free coordination position of the Cyt-a3, and efficiently inhibit the respiration. Therefore, both carbon monoxide and prussic acid (HCN) are very potent poisons.
Related Topics