Chapter: Basic Concept of Biotechnology : Biomolecules
Structure of MonosaccharideŌĆÖs
Structure of
MonosaccharideŌĆÖs
Although
a large number of monosaccharideŌĆÖs are found in nature, we will confine our
discussion here to four of them only viz. D-glucose, D-fructose, D-ribose and
2-deoxy-D-ribose. D-Glucose (an aldohexose) is the monomer for many other
carbohydrates. Alone or in combination, glucose is probably the most abundant
organic compound on the earth. D-Fructose (a ketohexose) is a sugar that is
found withglucose in honey and fruit juices. D-Ribose (an aldopentose) is found
in ribonucleic acids (RNA) while. 2-Deoxy-D-ribose is an important
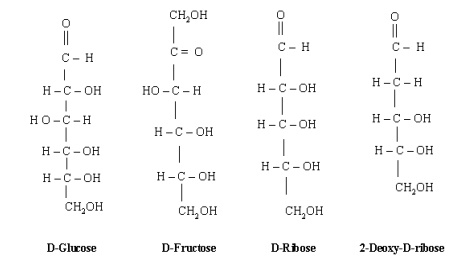
These
monosaccharides generally exist as cyclic compounds in nature. A ring is formed
by a reaction between the carbonyl group and one of the hydroxyl groups present
in the molecule. Glucose preferentially forms the six member rings which can be
in two different isomeric forms called ╬▒- and ├¤-forms (shown below as I &
II). The two forms differ only in the arrangement of the hydroxyl group at
carbon No.1. Such isomers are called anomers. Formation of these cyclic
structures (I and II) from the open chain structure can be shown as follows.
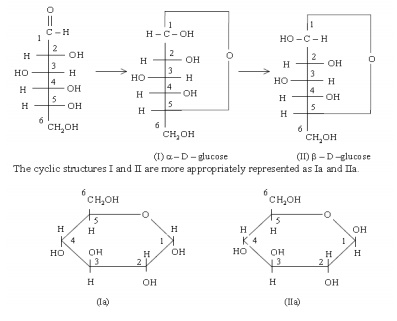
The
╬▒- and ├¤-forms of other sugars also exist in the cyclic form. D-Ribose forms a
five member ring structure as shown below
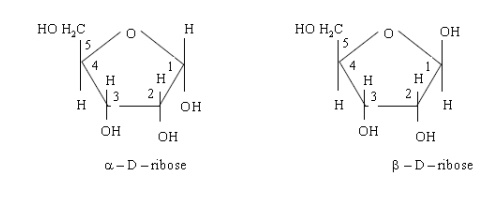
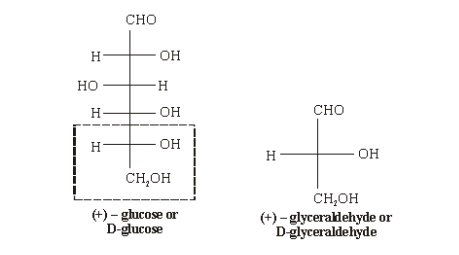
D-before the name of above example indicates the configuration of particular stereoisomer. Stereoisomers are assigned relative configurations as DŌĆō or L ŌĆō. This system of assigning the relative configuration refers to their relation with glyceraldehydes. Glyceraldehydes contain one asymmetric carbon atom so exists in two enantiomeric forms as shown below.
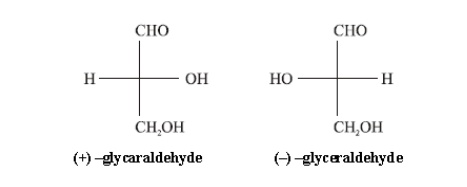
All
those compounds which can be correlated to (+) - glyceraldehyde are said to
have D-configuration and those can be correlated to (ŌĆō) -glyceraldehyde are
said to have LŌĆōconfiguration. In monosaccharides it is the lowest asymmetric
carbon atom (shown in the box) by which the correlation is made. As in (+)
glucose the lowest asymmetric carbon atom has ŌĆōOH group on the right side which
matches with (+) glyceraldehyde hence it is assigned D-configuration.
Related Topics