Chapter: Organic Chemistry: Organic spectroscopy and analysis
Proton nuclear magnetic resonance spectroscopy: Introduction
PROTON NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY
Key Notes
Introduction
Nmr
spectroscopy involves the detection of nuclei. Nuclei are charged spin-ning
bodies that have an associated magnetic moment. In proton nmr, an external
magnetic field is applied to force protons into two possible orien-tations
which are not of equal energy. Those nuclei spinning with their mag-netic
moments aligned with the field are more stable than those spinning with their
magnetic moments aligned against the field. There is a greater population of
nuclei in the more stable orientation. Applying energy in the form of
electromagnetic radiation causes the nuclei to flip or resonate between the two
orientations resulting in an overall absorption of energy. When the radiation
is stopped, the nuclei relax to the more stable popula-tion ratio resulting in
an emission of energy. A spectrum can be obtained by measuring the energy
absorbed or the energy emitted. The energy required for resonance is in the
radiofrequency region of the electromagnetic spectrum and is equal to the
frequency with which the magnetic moment precesses round the axis of the
applied magnetic field. The precessional frequency increases if the applied
magnetic field is increased. Nmr spectrometers and spectra are defined by the
energy required for resonance.
Introduction
Of all the spectroscopic methods, nuclear
magnetic resonance (nmr) spectroscopy is the most useful in determining the
structure and stereochemistry of organic compounds. Nmr spectroscopy detects
the nuclei of atoms in a molecule, and one of the most useful forms of nmr is
the detection of hydrogen atoms. The nucleu of a hydrogen atom is a single
proton and so the method is also known as protonnmr.
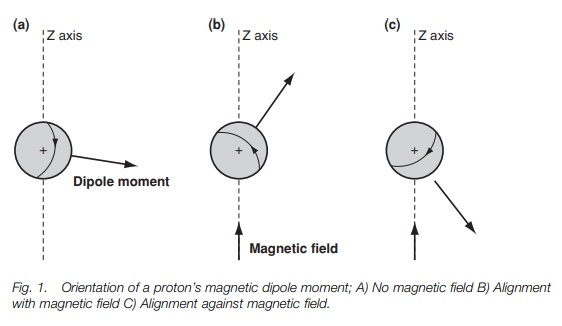
A proton spins around its axis, and whenever a charged body spins, a magnetic
field is set up which can be represented by a magnetic dipole moment (Fig. 1a). Under normal conditions, the
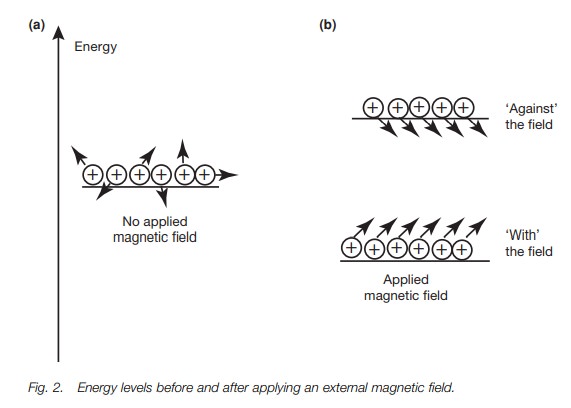
protons and their magnetic moments are randomly orientated and so there is no
overall magnetic field (Fig. 2a).
However, the situation changes if an external
magnetic field is applied to the sample. In Fig.
1b and c an external magnetic field has been applied in the direc- tion of
the z-axis. This field interacts with the magnetic moment of the nucleus,
forcing the nucleus to spin in only two possible orientations. In Fig. 1b, the nucleus is spinning
such that the
magnetic moment is
pointing in roughly
the same
The other orientation (Fig. 1c) has the nucleus spinning such
the dipole moment is pointed roughly against the field. Crucially, these two
ori-entations are not of equal energy. The orientation against the field is
less stable than the orientation with the field (Fig. 2b). This is crucial to understanding why we get an nmr
spectrum.
The energy difference between the two
orientations is extremely small and so the energy levels involved are almost
equally populated. However, there is a slight excess of nuclei in the more
stable energy level, and so if we were to pro-mote these nuclei to the higher
energy level, energy would be absorbed and a spectrum could be measured. Before
we look at what energy is required, we shall return to our picture of the
spinning nucleus. Fig. 1b shows the
nucleus spinning with the field – the more stable orientation. Notice that the
dipole moment is not directly aligned with the magnetic field, but is at an
angle to it. This means that the dipole moment experiences a force or a torque,
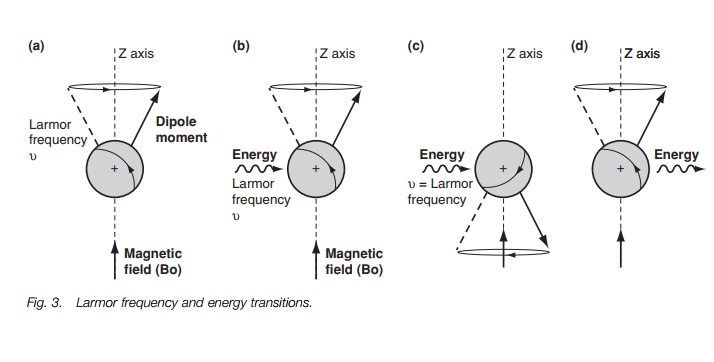
which causes it to rotate or pre-cess around the z-axis (Fig. 3a) like a gyroscope. A gyroscope is a spinning body which,
when set at an angle to the vertical axis of gravity, precesses round that
vertical axis. The nucleus is undergoing exactly the same kind of motion. It
too is a spinning body but it precesses round the axis of an applied magnetic
field. The rate at which the dipole moment precesses round the z-axis is called
the Larmorfrequency and is dependant
on the strength of the applied magnetic field. If themagnetic field increases,
the rate of precession increases.
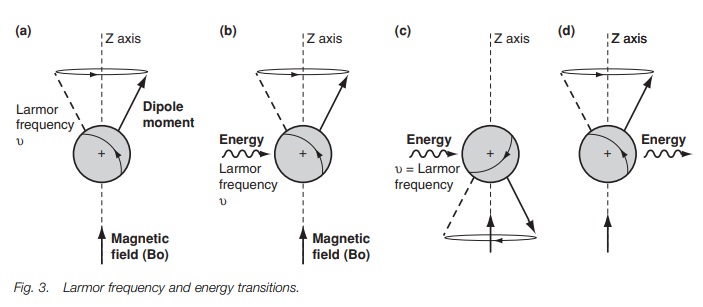
In order to get a spectrum we need to get
transitions between the two energy levels. This can be achieved by firing in a
burst of energy in the form of electro-magnetic radiation (Fig. 3b). The effect of this energy is to excite the nucleus and to
cause it to ‘flip’ such that it is now against the magnetic field (Fig. 3c). This orientation is less
stable than the original orientation and so the nucleus has absorbed energy. It
is found that the energy required to do this has the same frequency as the
Larmor frequency. If the electromagnetic radiation is now stopped, the nucleus
relaxes back to the more stable orientation (Fig. 3d). As a result, energy is emitted. Such energy can be
detected and measured leading to a signal in an nmr spectrum. The energy
difference between the two orientations is extremely small and is in the
radiofrequency region of the electromagnetic spectrum. The energy difference is
proportional to the Larmor frequency, which is proportional to the strength of
the external applied magnetic field. In a magnetic field of 14 100G, the energy
difference is 60 × 106 Hz or 60 MHz. In a magnetic
field of 23 500G the energy difference is 100 MHz. NMR spectrometers work at a
specific magnetic field and thus a specific electromagnetic radiation is
required for resonance. The convention is to identify the spectrometers and
their spectra by the strength of the electromagnetic radiation used (i.e. 60
MHz or 100 MHz).
Related Topics