Chapter: Obstetrics and Gynecology: Assessment of Genetic Disorders in Obstetrics and Gynecology
Prenatal Screening
PRENATAL SCREENING
Obstetricians are responsible for
determining if a woman is at increased risk for fetal abnormalities, and for
describ-ing and offering appropriate prenatal screening or diag-nostic tests. The purpose of prenatal genetic screening is
todefine the risk for a genetic disease in a low-risk population.
screening
test differs from a
diagnostic test in thatscreening
tests only assess the risk that a child will have a genetic disease; they
cannot confirm or rule out the presence of the dis-ease. A diagnostic test is
given if a screening test is positive, to assess whether the disease is present
or absent in the developing fetus. Genetic screening tests are routinely
offered to allwomen to detect neural tube defects (NTDs), Down syn-drome, and
trisomy 18. In addition, individuals of certain ethnic groups can be tested to
detect whether they carry a gene for a particular disorder.
First-Trimester Screening
First-trimester screening tests are used to assess the risk of Down syndrome, trisomy 18, and trisomy 13 in a devel-oping fetus. First-semester serum screening for Down syndrome consists of tests for levels of two biochemical markers: free or total human chorionic gonadotropin(hCG) and pregnancy-associated plasma protein A (PAPP-A). An elevated level of hCG (1.98 of the medianobserved in euploid pregnancies [MoM]) and a decreased level of PAPP-A (0.43 MoM) have been associated with Down syndrome. An ultrasonographic marker for Down syndrome is the size of the nuchal transparency (NT), a fluid collection at the back of the fetal neck that can be seen between 10 and 14 weeks of gestation (Fig. 7.5). An increase in the size of the NT between 10 4/7 and 13 6/7 weeks of gestation is recognized to be an early presenting feature of a variety of chromosomal, genetic, and struc-tural abnormalities. When used alone, NT measurement has a detection rate for Down syndrome of 64% to 70%. Combining NT measurement with the other first-trimester biochemical markers yields an 82% to 87% detection rate of Down syndrome, with a 5% false-positive rate, which of her pregnancy.
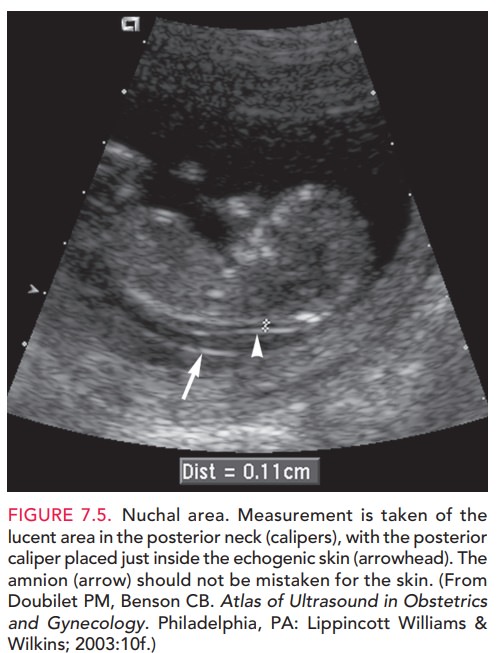
Women who have
had first-trimester screening for aneuploidy should not undergo independent
second-trimester serum screening in the same pregnancy. When these test results
are interpreted independently, the false-positive rates are additive, leading
to many more unnecessary invasive procedures (11% to 17%). After
first-trimester screening, subsequent second-trimester Down syndrome screening
is not indicated, unless it is being performed as a component of an integrated
test (explained below), stepwise sequential, or contingent sequential test.
TRIPLE AND QUADRUPLE SCREENING TESTS
An association between low
maternal serum alpha-fetoprotein (AFP) levels
and Down syndrome wasreported in 1984. In the 1990s, hCG and unconjugated
estriol were used in combination with maternal serum AFP to improve the
detection rates for Down syndrome and trisomy 18. The average maternal serum AFP
level in Down syndrome pregnancies is reduced to 0.74 MoM. Intact hCG is
increased in affected pregnancies, with an average level of 2.06 MoM, whereas
unconjugated estriol is reduced to an average level of 0.75 MoM. When the lev-els of all three markers (triple screen) are used to modify the
maternal age-related Down syndrome risk, the detection rate for Down syndrome
is approximately 70%; approximately 5% of all pregnancies will have a positive
screen result. Typically,the levels of all three markers are reduced when
the fetus has trisomy 18. Adding inhibin
A to the triple screen (quad-ruple
screen) improves the detection rate for Down syndrometo approximately 80%. The
median value of the maternalinhibin A level is increased at 1.77 MoM in Down
syn-drome pregnancies, but inhibin A is not used in the calcu-lation of risk
for trisomy 18. These biochemical screening tests are performed at 15 to 20
weeks of gestation.
ULTRASOUND SCREENING
In the
second trimester, gross abnormalities, such as cardiac defects, as well as a
group of subtle sonographic markers (soft markers) may be associated with an
increased risk for Down syndrome in certain women (Box
7.3). Although findings of soft markers donot significantly increase the risk
of Down syndrome, they should be considered in the context of first-trimester
screening results, patient’s age, and history. The signifi-cance of
ultrasonographic markers identified by a second-trimester ultrasound
examination in a patient who has had a negative first-trimester screening test
result is unknown. Studies indicate that the highest detection rate is achieved
with systematic combination of ultrasonographic markers and gross anomalies,
such as thick nuchal fold or cardiac defects. However, an abnormal
second-trimester ultrasound finding identifying a major congenital anomaly
significantly increases the risk of aneuploidy and warrants further coun-seling
and the offer of a diagnostic procedure.
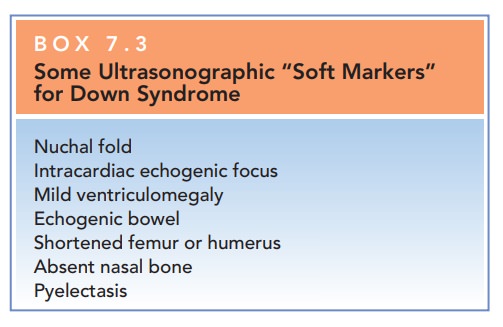
Box 7.3
Some Ultrasonographic “Soft Markers” for Down Syndrome
Nuchal fold
Intracardiac echogenic focus
Mild ventriculomegaly
Echogenic bowel
Shortened femur or humerus
Absent nasal bone
Pyelectasis
SCREENING FOR NTDS
Maternal serum AFP is also used to screen for NTDs, congenital structural abnormalities of the brain and vertebral column. NTDs occur in approximately 1.4–2 per 1000 births in the United States and are the second most common major con-genital abnormality worldwide (cardiac malformations are the most common). Maternal serum AFP evaluation is an effective screening test for NTDs and should be offered to all pregnant women, unless they plan to have amniotic AFP measurement as part of prenatal diagnosis for chromosomal abnormalities or other genetic diseases. Most affected preg-nancies can be identified by an elevated maternal serum AFP level, defined as 2.5 MoM for a singleton pregnancy. Womenwith a positive screening test should receive an ultrasound examination to detect identifiable causes of false-positive results (e.g., fetal death, multiple gestation, underestimate of gestational age) and for targeted study of fetal anatomy for NTDs and other defects associated with elevated mater-nal serum AFP.
Approximately 90% of newborns
with NTDs are born to women not offered amniocentesis because they have no
family or medication history that would indicate them to be at increased risk. Folic acid has been shown to prevent
recurrence and occurrence of neural tube defects. Becausemost individuals at increased risk do not know it until they
have an affected child, all women should be advised to take a vitamin that
contains at least 0.4 mg of folic acid prior to conception. Forwomen who
previously have had a child with a neural tube defect, the recommended dose is
4 mg daily.
Integrated Screening
The results of both
first-trimester and second-trimester screening and ultrasound can be combined
to increase their ability to detect Down syndrome. This“integrated”approach to
screening uses both the first-trimester and second-trimester markers to adjust
a woman’s age-related risk of having a child with Down syndrome. The
results are reported onlyafter both first-trimester and second-trimester
screening tests are completed. Integrated screening provides the highest
sensitivity with the lowest false-positive rate. The lower false-positive rate
results in fewer invasive tests and, thus, fewer procedure-related losses of
normal pregnancies. Although some patients value early screening, others are
willing to wait several weeks if doing so results in an improved detection rate
and less chance that they will need an invasive diagnostic test. Concerns about
integrated screening include possible patient anxiety generated by hav-ing to
wait 3 to 4 weeks between initiation and comple-tion of the screening and the
loss of the opportunity to consider CVS if the first-trimester screening
indicates a high risk of aneuploidy.
Related Topics