Chapter: Mechanical : Engineering Thermodynamics : The Second Law of Thermodynamics
Entropy
ENTROPY
The first law of
thermodynamics deals with the property energy and the conservation of energy.
The second law introduced in the previous chapter, leads to the definition of a
new property called entropy. Entropy is defined in terms of a calculus
operation, and no direct physical picture of it can be given. In this chapter,
Clausius inequality, which forms the basis for the definition of entropy will
be discussed first. It will be followed by the discussion of entropy changes
that take place during various processes for different working fluids. Finally,
the reversible steady-flow work and the isentropic efficiencies of various
engineering devices such as turbine and compressors will be discussed.
The
Clausius Inequality
Consider
two heat engines operating between two reservoirs kept at temperature TH
and TL as shown in the Figure 5.1. Of the two heat engines, one is
reversible and the other is irreversible.
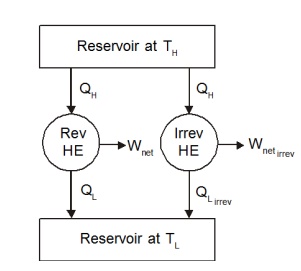
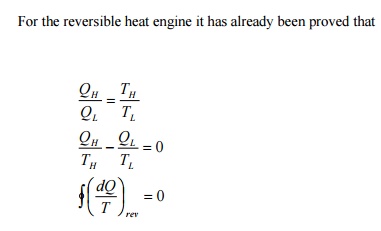
As discussed earlier,
the work output from the irreversible engine should be less than that of the
reversible engine for the same heat input QH. Therefore QL,Irrev
will be greater than QL,Rev . Let us define
QL,Irrev
=QL,Rev +dQ
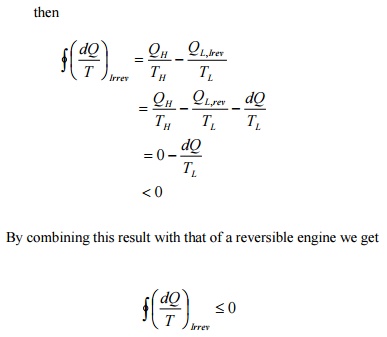
This
is known as Clausius inequality.
Clausius
inequality forms the basis for the definition of a new property known as
entropy.
Consider
a system taken from state 1 to state 2 along a reversible path A as shown in
Figure 5.2. Let the system be brought back to the initial state 1 from state 2
along a reversible path B. Now the system has completed one cycle. Applying
Clausius inequality we get
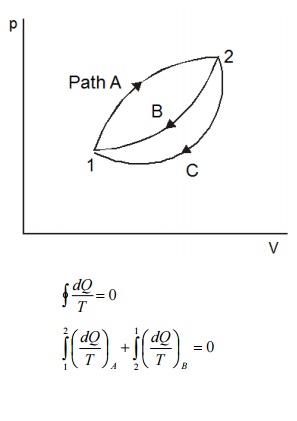
Instead
of taking the system from state2 to state1 along B, consider another reversible
path C. Then for this cycle 1-A-2-C-1, applying Clausius inequality :
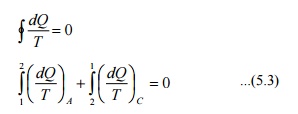
Comparing 5.2 & 5.3
Hence,
it can be concluded that the quantity is a point function, independent of the
path followed. Therefore it is a property of the system. Using the symbol S for
entropy we can write
upon
integration we get

For
a reversible process.
Entropy
change for an irreversible process
The
relationship between the entropy change and heat transfer across the boundary during
an irreversible processes can be illustrated with a simple cycle composed of
two processes, one of which is internally reversible and the other is
irreversible, as shown in Figure 5.3. The Clausius inequality applied to this
irreversible cycle can be written as
Since
the process B is internally reversible, this process can be reversed, and
therefore
As
defined in equation 5.5, since the process B being reversible the integral on
the left hand side can be expressed as
Temperature - Entropy diagram
In a T-s diagram
consider a strip of thickness ds with mean height T as shown in Figure 5.4.
Then Tds gives the area of the strip.
For
a reversible process the elemental heat transfer
dQ
=Tds =Area
of the strip
To
get the total heat transfer the above equation should be integrated between the
limits 1 and 2, so that, we get
This is equivalent to
the area under a curve representing the process in a T-S diagram as shown in the
Fig 5.4.
Note: For
an isothermal process S2 -S1 =.
For reversible
adiabatic process S2 -S1
=0.
Change in Entropy
a) Solids and Liquids
Change
in entropy
Where dq =du + pdv
For
solids and liquids
pdv
= 0
Where
c- is the specific heat
b) For ideal gases change in entropy
Substituting
du
=Cv dT
Substituting dh =Cp dT
and We get
Upon
integration
Principle of Increase in Entropy
Applying
Clausius inequality,
For
an isolated system undergoing a process
Consider a system
interacting with its surroundings. Let the system and its surroundings are
included in a boundary forming an isolated system. Since all the reactions are
taking place within the combined system, we can express
or
Whenever a process
occurs entropy of the universe (System plus surroundings) will increase if it
is irreversible and remain constant if it is reversible. Since all the
processes in practice are irreversible, entropy of universe always increases
ie., (Ds)universe>0
This
is known as principle of increase of entropy.
Adiabatic Efficiency of Compressors and
Turbines
In steady flow
compressors and turbines reversible adiabatic process is assumed to be the
ideal process. But due to the irreversibilities caused by friction between the
flowing fluid and impellers, the process is not reversible though it is
adiabatic. Percentage deviation of this process from the ideal process is
expressed in terms of adiabatic efficiency.
(a) Compressors :
Since compressors are
work consuming devices actual work required is more than ideal work. For
compressors handling ideal gases
(b) Turbines :
In turbine due to irreversibilities the actual work
output is less than the isentropic work.
Solved Problems
Prob : 5.1 A
body at 200oC undergoes an reversible isothermal process. The heat energy
removed in the process is 7875 J. Determine the change in the entropy of the
body.
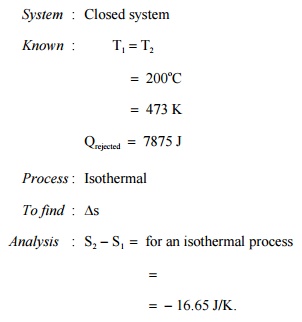
Comment : Entropy
decreases as heat is removed from the system.
Prob : 5.2 A
mass of 5 kg of liquid water is cooled from 100oC to 20oC. Determine the change
in entropy.
System : Closed system
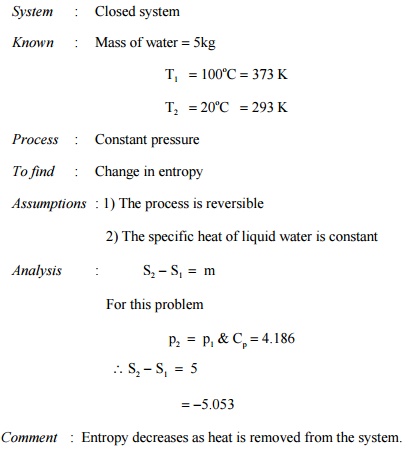
Comment :
Entropy decreases as heat is removed from the system.
Prob : 5.3
Air is compressed isothermally from 100 kPa to 800
kPa. Determine the change in specific entropy of the air.
System : Closed/Open
Known : p1 =100 kPa
p2
=800
kPa
To
find : DS - change in Specific entropy
Analysis : DS = -R ln [Since the process is isothermal]
= 0 .287- x ln
= 0.597 -kJ/kgK.
Prob : 5.4
A mass of 5 kg of air is compressed from 90 kPa, 32oC
to 600 kPa in a polytropic process, pV1.3=constant. Determine the change entropy.
System : Closed / Open
Known : p1 =90 kPa
T1
=32oC =305 K
p2
=600
kPa
m
=5
kg
Process
: pV1.3 =Constant
To
find : DS - Change in entropy
Analysis :
S2 -S1 =m
Where
T2 =T1
=305
=473 K
\ S2 -S1 =5
=-0.517 kJ/K.
Comment
: For air the ratio of Cp and Cv
is equal to 1.4. Therefore the polytropic index
n -1.3(<1.4) indicates that some heat is removed from the system
resulting in negative entropy.
Prob : 5.5
A rigid insulated container holds 5 kg of an ideal
gas. The gas is stirred so that its state changes from 5 kPa and 300 K
to 15 kPa. Assuming Cp =1.0
kJ/kgK and g =1.4,
determine the change of entropy of the system.
System : Closed
Process
: Constant volume since the gas is stirred in
an rigid container
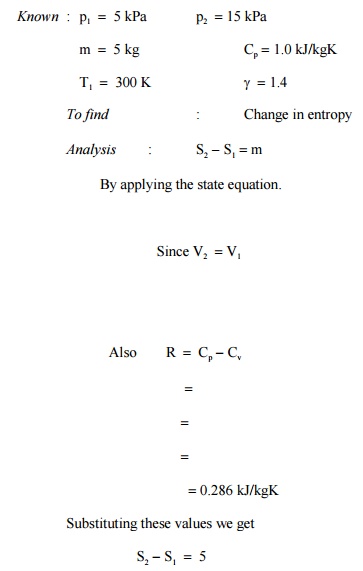
3 .922 kJ/K =
Comment :
Though this process is adiabatic it is not isentropic since the process of
stirring is an irreversible process.
Prob : 5.6
An insulated rigid vessel is divided into two
chambers of equal volumes. One chamber contains air at 500 K and 2 MPa.
The other chamber is evacuated. If the two chambers are connected d, what would
be the entropy change ?
System : Closed system
Process
: Unresisted expansion
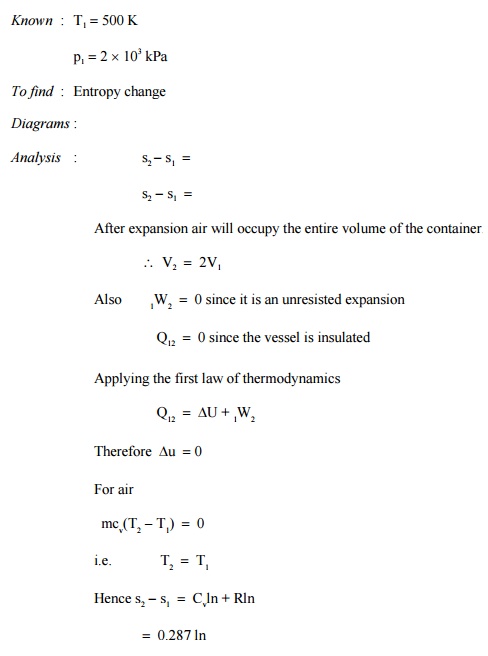
=0.199 kJ/kgK
Comment : Though the process is adiabatic entropy
increases as the process involving
unresisted expansion is an irreversible process. It
also proves the fact
Prob : 5.7
An adiabatic chamber is partitioned into two equal
compartments. On one side there is oxygen at 860 kPa and 14oC.
On the other side also, there is oxygen, but at 100 kPa and 14oC.
The chamber is insulated and has a volume of 7500 cc. The partition is abruptly
removed. Determine the final pressure and the change in entropy of the
universe.

Prob : 5.8
A closed system is taken through a cycle consisting
of four reversible processes. Details of the processes are listed below.
Determine the power developed if the system is executing 100 cycles per
minutes.
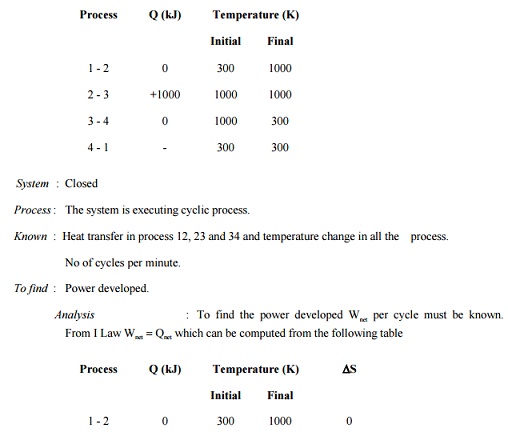
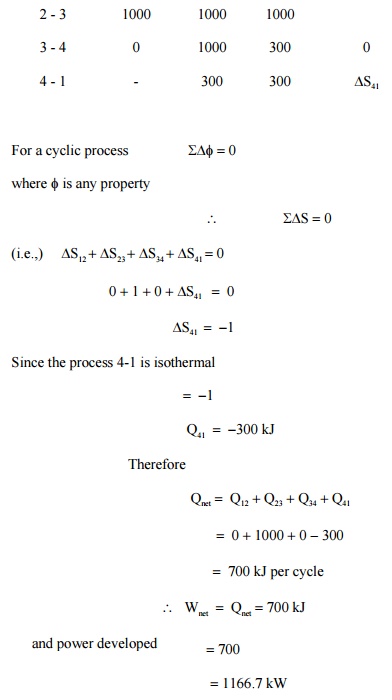
Related Topics