Chapter: Organic Chemistry: Alcohols, phenols, and thiols
Chemistry of thiols
CHEMISTRY OF THIOLS
Key Notes
Preparation
Thiols
can be prepared by the reaction of an alkyl halide with KOH and an excess of
hydrogen sulfide. A hydrogen sulfide anion is formed which undergoes an SN2
reaction with the alkyl halide. Hydrogen sulfide has to be in excess in order
to limit further reaction to a thioether. Alternatively, the alkyl halide can
be treated with thiourea to form an S-alkylisothiouronium
salt which is then hydrolyzed with aqueous base to give the thiol. Disulfides
can be reduced to thiols with zinc and acid.
Properties
Hydrogen
bonding is weak, resulting in boiling points which are lower than comparable
alcohols and similar to comparable thioethers.
Reactivity
Thiols
(RSH) contain a large polarizable sulfur atom. The S–H bond is weak compared to
alcohols, making thiols prone to oxidation. Thiolate ions are extremely good
nucleophiles whilst being weak bases. Thiols are stronger acids than alcohols.
Reactions
Thiols
are oxidized by bromine or iodine to give disulfides. Treatment of a thiol with
a base results in the formation of a thiolate ion.
Preparation
Thiols can be prepared by the treatment of
alkyl halides with an excess of KOH and hydrogen sulfide (Fig. 1a). The preparation is an SN2 reaction involving
the generation of a hydrogen sulfide anion (HS-) as nucleophile. A
problem with this reaction is the possibility of the product being ionized and
reacting with a second molecule of alkyl halide to produce a thioether (RSR) as
a byproduct. An excess of hydrogen sulfide is normally used to avoid this
problem.
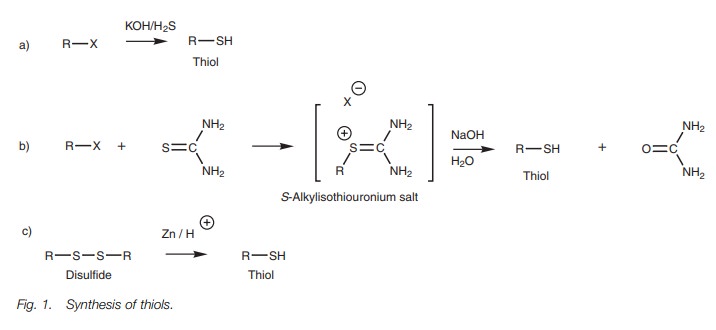
The problem of thioether formation can also be
avoided by using an alternative procedure involving thiourea (Fig. 1b). The thiourea acts as the
nucleophile in an SN2 reaction to produce an S-alkylisothiouronium salt which is then hydrolyzed with aqueous
base to give the thiol.
Thiols can also be formed by reducing
disulfides with zinc in the presence of acid (Fig. 1c).
Properties
Thiols form extremely weak hydrogen bonds –
much weaker than alcohols – and so thiols have boiling points which are similar
to comparable thioethers and which are lower than comparable alcohols. For
example, ethanethiol boils at 37°C
whereas ethanol boils at 78°C.
Low molecular weight thiols are notorious for
having disagreeable aromas.
Reactivity
Thiols are the sulfur equivalent of alcohols
(RSH). The sulfur atom is larger and more polarizable than oxygen which means
that sulfur compounds as a whole are more powerful nucleophiles than the
corresponding oxygen compounds. Thiolate ions
(e.g. CH3CH2S-
) are
stronger nucleophiles and
weaker bases than corresponding alkoxides (CH3CH2O-
). Conversely, thiols are stronger acids than corresponding alcohols.
The relative size difference between sulfur and oxygen also means that sulfur’s bonding orbitals are more diffuse than oxygen’s bonding orbitals. This results in a poorer bonding interaction between sulfur and hydrogen, than between oxygenand hydrogen. As a result, the S–H bond of thiols is weaker than the O–H bond of alcohols (80 kcal mol-1 vs. 100 kcal mol -1). This in turn means that the S–H bond of thiols is more prone to oxidation than the O–H bond of alcohols.
Eactions
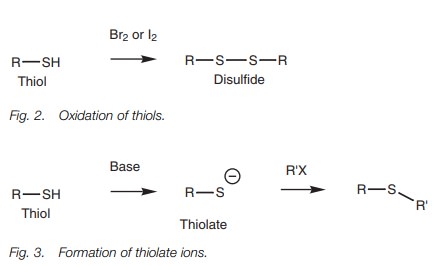
Thiols are easily oxidized by mild oxidizing
agents such as bromine or iodine to give disulfides (Fig. 2).
Thiols react with base to form thiolate ions
which can act as powerful nucleo- philes.
Related Topics