Chapter: Organic Chemistry: Ethers, epoxides, and thioethers
Preparation of ethers, epoxides, and thioethers
PREPARATION OF ETHERS, EPOXIDES, AND THIOETHERS
Key Notes
Ethers
Ethers
can be prepared by the SN2 reaction of an alkyl halide with an
alkoxide ion. The reaction works best for primary alkyl halides. Alcohols and
alkyl halides can be reacted in the presence of silver oxide to give an ether.
Alkenes can be treated with alcohols in the presence of mercuric
trifluoroacetate to form ethers by electrophilic addition.
Epoxides
Epoxides
can be synthesized from alkenes and meta-chloroperbenzoic
acid, or by converting the alkene to a halohydrin and treating the product with
base to induce an intramolecular SN2 reaction which displaces the
halogen atom. Aldehydes and ketones can be converted to epoxides on treatment
with a sulfur ylide.
Thioethers
Thioethers
are prepared by the SN2 reaction between an alkyl halide and a
thiolate ion. Symmetrical thioethers can be prepared by treating the alkyl
halide with KOH and hydrogen sulfide where the latter is not in excess.
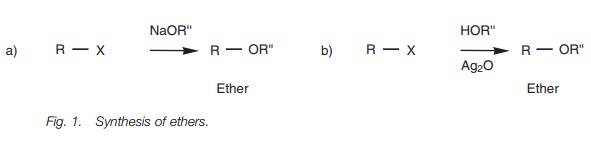
Ethers
The Williamson ether synthesis is the best
method of preparing ethers (Fig. 1a).
The procedure involves the SN2 reaction
between a metal alkoxide and a primary alkyl
halide or tosylate.
The alkoxide required
for the reaction
is prepared by treating an alcohol with a strong base such as sodium
hydride. An alternative procedure is to treat the alcohol directly with the
alkyl halide in the presence of silver oxide, thus avoiding the need to prepare
the alkoxide beforehand (Fig. 1b).
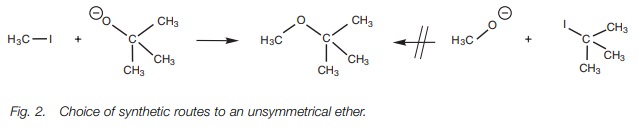
If an unsymmetrical ether is being synthesized,
the most hindered alkoxide should be reacted with the simplest alkyl halide,
rather than the other way roundZ (Fig. 2).
Since this is an SN2 reaction, primary alkyl halides react better
than secondary or tertiary alkyl halides.
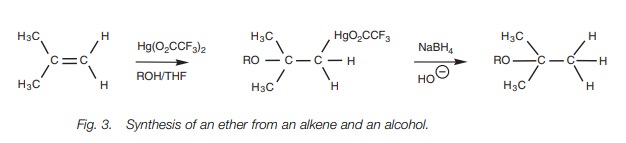
Alkenes can be converted to ethers by the electrophilic
addition of mercuric trifluoroacetate, followed by addition of an alcohol. An
organomercuric inter-mediate is obtained which can be reduced with sodium
borohydride to give the ether.
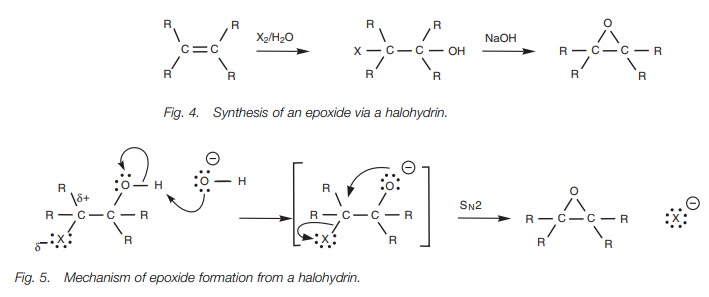
Epoxides
Epoxides can be synthesized by treating
aldehydes or ketones with sulfur ylides.
They can also
be prepared from
alkenes by reaction
with m- chloroperoxybenzoic acid
. Alternatively they can be obtained from alkenes in a two-step process (Fig.
4). The first step involves electrophilic addition of a halogen in aqueous
solution to form a halohydrin. Treatment
of the halohydrin with base then ionizes the alcohol group, which can then act
as a nucleophile (Fig. 5). The oxygen uses a lone pair of electrons to form a bond
to the neighboring electrophilic
carbon, thus displacing
the halogen by an
intramolecular SN2 reaction.
Thioethers
Thioethers (or sulfides) are prepared by the SN2 reaction of primary or secondary alkyl halides with a thiolate anion (RS-),. The reaction is similar to the Williamson ether synthesis.
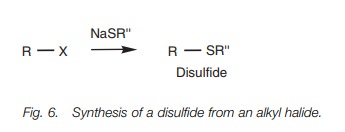
Symmetrical thioethers can be prepared by
treating an alkyl halide with KOH and an equivalent of hydrogen sulfide. The
reaction produces a thiol which is ionized again by KOH and reacts with another
molecule of alkyl halide.
Related Topics