Chapter: Organic Chemistry: Ethers, epoxides, and thioethers
Properties of ethers, epoxides, and thioethers
PROPERTIES OF ETHERS, EPOXIDES AND THIOETHERS
Key Notes
Ethers
Ethers
consist of ansp3 hybridized
oxygen linked to two carbon atoms by a single σ bond. Alkyl ethers are ethers where two alkyl
groups are linked to the oxygen. Aryl ethers are ethers where one or two
aromatic rings are attached to the oxygen. Since ethers cannot form hydrogen
bonds, they have lower boiling points than comparable alcohols, and similar
boiling points to comparable alkanes. However, hydrogen bonding is possible
with protic solvents which means that ethers have water solubilities similar to
alcohols of equivalent molecular weight. Ethers are relatively unreactive since
they have weak nucleophilic and electrophilic centers.
Epoxides
Epoxides
are three-membered cyclic ethers which are more reactive than other cyclic or
acyclic ethers due to the ring strain inherent in three-membered rings. They
will react with nucleophiles by an SN2 reaction at the electrophilic
carbons.
Thioethers
Thioethers
are the sulfur equivalents of ethers. The polarizable sulfur can stabilize a
negative charge on an adjacent carbon making protons attached to that carbon
acidic.
Spectroscopic analysis of ethers and epoxides
The
presence of an ether or epoxide is indicated by C–O stretching absorp-tions in
the IR spectrum. Supporting evidence can be obtained from the chemical shifts
of neighboring groups in the 1H and 13C nmr spectra. It
is important to consider other spectroscopic evidence as well as the molecular
formula before deciding whether an ether or epoxide is present.
Ethers
Ethers consist of an oxygen linked to two
carbon atoms by σ bonds. In aliphatic ethers (ROR), the three
atoms involved are sp3
hybridized and have a bond angle of 112°. Aryl ethers are ethers where the oxygen is
linked to one or two aromatic rings (ArOR or ArOAr) in which case the attached
carbon(s) is sp2
hybridized.
The C–O bonds are polarized such that the
oxygen is slightly negative and the carbons are slightly positive. Due to the
slightly polar C–O bonds, ethers have a small dipole moment. However, ethers
have no X–H groups (X heteroatom) and cannot interact by hydrogen bonding.
Therefore, they have lower boiling points than comparable alcohols and similar
boiling points to comparable alkanes. How-ever, hydrogen bonding is possible to
protic solvents resulting in solubilities similar to alcohols of comparable
molecular weight.
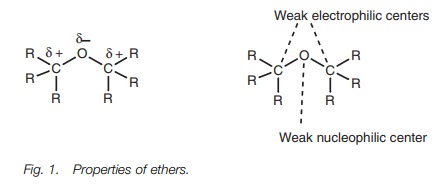
The oxygen of an ether is a nucleophilic center and the neighboring carbons are electrophilic centers, but in both cases the nucleophilicity or electrophilicity is weak (Fig. 1). Therefore, ethers are relatively unreactive.
Epoxides
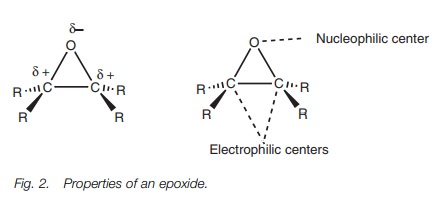
Epoxides (or oxiranes) are three-membered cyclic ethers and differ from other cyclic and acyclic ethers in that they are reactive to various reagents. The reason for this reactivity is the strained three-membered ring. Reactions with nucleophiles can result in ring opening and relief of strain. Nucleophiles willattack either of the electrophilic carbons present in an epoxide by an SN2 reaction (Fig. 2).
Thioethers
Thioethers (or sulfides; RSR) are the sulfur
equivalents of ethers (ROR). Since the sulfur atoms are polarizable, they can
stabilize a negative charge on an adjacent carbon atom. This means that
hydrogens on this carbon are more acidic than those on comparable ethers.
Spectroscopic analysis of ethers and epoxides
The IR spectra of ethers are characterized by
C–O stretching absorptions. An aliphatic ether tends to have an absorption in
the region 1150–1070 cm−1 which
is often stronger than surrounding peaks.
Alkyl aryl ethers
tend to give
two relatively strong absorptions, one in the region 1275–1200 cm−1 and the other in the region 1075–1020 cm−1. The C–O stretching absorption for epoxides
occurs in the region 1260–1200 cm−1. C–O Stretching absorptions are also possible
for carboxylic acids and esters, as well as for alcohols and phenols. Therefore
it is important to consider other evidence before deciding whether an ether or
epoxide is present. For example, if the molecular formula only has one oxygen,
this rules out the possibility of an acid or an ester. If there are no D2O
exchangeable protons in the 1H nmr spectrum, this rules out alcohols
and phenols.
The 1H and 13C nmr
spectra of an ether do not give direct evidence of the func-tional group but
may indicate its presence indirectly by the chemical shifts of neighboring
groups. For example, the methyl group of a methyl ether appears at 3.3 ppm in
the 1H spectrum and at 59 ppm in the 13C spectrum.
The protons of an epoxide show characteristic
signals at 2.5–3.5 ppm in the 1H nmr spectrum.
Related Topics