Chapter: Organic Chemistry: Alcohols, phenols, and thiols
Reactions of alcohols
REACTIONS OF ALCOHOLS
Key Notes
Acid–base reactions
Alcohols
are weak acids and react with strong bases to form an alkoxide ion.
Elimination
Alcohols
are dehydrated to alkenes by heating with sulfuric acid. The reac-tion involves
an E1 mechanism through an intermediate carbocation, and so tertiary alcohols
react better than secondary alcohols, and secondary alco-hols react better than
primary alcohols. If a choice of alkenes is possible, the most substituted
alkene is preferred. Dehydration of secondary and tertiary alcohols is also
possible under milder basic conditions using POCl3. The reaction
takes place by an E2 mechanism.
Synthesis of alkyl halides
Tertiary
alcohols and some secondary alcohols are converted to alkyl chlo-rides and alkyl
bromides on treatment with HCl and HBr respectively. The mechanism involves
protonation of the hydroxyl group to turn it into a good leaving group, then a
normal SN1 reaction. Primary and secondary alcohols are converted to
alkyl chlorides and alkyl bromides by an SN2 reac-tion involving
thionyl chloride and phosphorus tribromide respectively. The reagents serve to
convert the hydroxyl group into a better leaving group and also act as a source
of the halide ion.
Synthesis of mesylates and tosylates
Alcohols
can be treated with sulfonyl chlorides to give structures known as sulfonates.
Two common examples are mesylates and tosylates. The mesyl-ate and tosylate
groups are excellent leaving groups and these compounds undergo the SN2
reaction in the same way as alkyl halides. Mesylates and tosylates serve to
convert the hydroxyl group of an alcohol from a poor leaving group into a very
good leaving group.
Oxidation
Primary
alcohols are oxidized to aldehydes with pyridinium chlorochro- mate (PCC) in
dichloromethane, and oxidized to carboxylic acids with CrO3 in
aqueous acid. The former reaction stops at the aldehyde since the PCC is a mild
oxidizing agent and the reaction is carried out in dichloromethane.Under
aqueous acidic conditions with CrO3
as the oxidizing agent, the primary alcohol is converted to an aldehyde
which is then hydrated and oxidized again to the carboxylic acid. Secondary
alcohols are oxidized to ketones while tertiary alcohols are resistant to
oxidation.
Esterification
Alcohols
can be converted to esters by reaction with carboxylic acids, acid chlorides or
acid anhydrides.
Acid–base reactions
Alcohols are slightly weaker acids than water
which means that the conjugate base generated from an alcohol (the alkoxide ion) is a stronger base than
the con- jugate base of water (the hydroxide ion). As a result, it is not
possible to generate an alkoxide ion using sodium hydroxide as base. Alcohols
do not react with sodium bicarbonate or amines, and a stronger base such as
sodium hydride or sodium amide is required to generate the alkoxide ion (Fig. 1). Alcohols can also be converted
to alkoxide ions on treatment with potassium, sodium, or lithium metal. Some
organic reagents can also act as strong bases, for example Grignard reagents
and organolithium reagents.
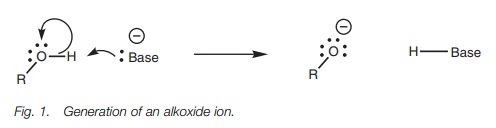
Alkoxide ions are neutralized in water and so
reactions involving these reagents should be carried out in the alcohol from
which they were derived, that is reac-tions involving sodium ethoxide are best
carried out in ethanol. Alcohols have a typical pKa of 15.5–18.0 compared to pKa values of 25 for ethyne, 38 for ammonia and 50 for
ethane.
Elimination
Alcohols, like alkyl halides, can undergo
elimination reactions to form alkenes. Since water is eliminated, the reaction
is also known as a dehydration.
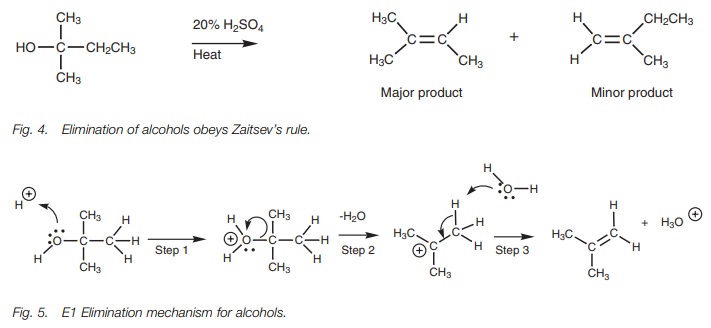
Like alkyl halides, the elimination reaction of
an alcohol requires the presence of a susceptible proton at the β-position (Fig. 3).
Whereas the elimination of alkyl halides is carried out under basic conditions, the elimination of alcohols is carried out under acid conditions. Under basic con-ditions, an E2 elimination would require the loss of a hydroxide ion as a leaving group. Since the hydroxide ion is a strong base, it is not a good leaving group and so the elimination of alcohols under basic conditions is difficult to achieve.
Elimination under acidic conditions is more
successful since the hydroxyl group is first protonated and then departs the
molecule as a neutral water molecule (dehydration)
which is a much better leaving group. If different isomeric alkenes are
possible, the most substituted alkene will be favored – another example of
Zaitsev’s rule (Fig. 4). The reaction
works best with tertiary alcohols since the elimination proceeds by the E1
mechanism.
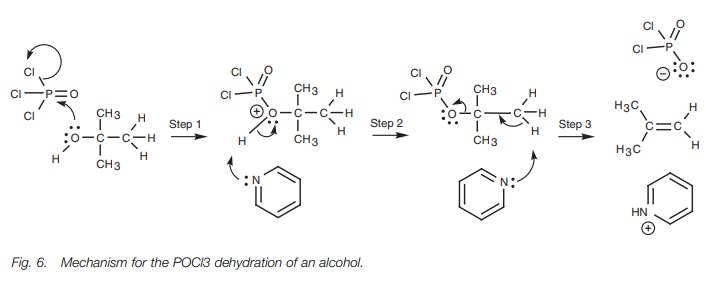
The mechanism (Fig. 5) involves the nucleophilic oxygen of the alcohol using one
of its lone pairs of electrons to form a bond to a proton to produce a charged
intermediate (Step 1). Now that the oxygen is protonated, the molecule has a
much better leaving group since water can be ejected as a neutral molecule. The
E1 mechanism can now proceed as normal. Water is lost and a carbocation is
formed (Step 2). Water then acts as a base in the second step, using one of its
lone pairs of electrons to form a bond to the β-proton of the carbocation. The C–H bond is broken and both the
electrons in that bond are used to form a π bond between the two carbons. Since this is an E1 reaction,
tertiary alcohols react better than pri-mary or secondary alcohols.
The E1 reaction is not ideal for the
dehydration of primary or secondary alco-hols since vigorous heating is
required to force the reaction and this can result in
Therefore, alternative
methods are useful. Reagents such as phosphorus oxychloride (POCl3)
dehydrate secondary and tertiary alcohols under mild basic conditions using
pyridine as solvent (Fig. 6). The
phosphorus oxychloride serves to activate the alcohol, converting the hydroxyl
function into a better leaving group. The mechanism involves the alcohol acting
as a nucleophile in the first step. Oxygen uses a lone pair of electrons to
form a bond to the elec-trophilic phosphorus of POCl3 and a chloride
ion is lost (Step 1). Pyridine then removes a proton from the structure to form
a dichlorophosphate intermediate (Step 2). The dichlorophosphate group is a
much better leaving group than the hydroxide ion and so a normal E2 reaction
can take place. Pyridine acts as a base to remove a β-proton and as this is happening, the electrons from the old C–H
bond are used to form a π bond and eject the leaving group (Step 3).
Synthesis of alkyl halides
Tertiary alcohols can undergo the SN1
reaction to produce tertiary alkyl halides. Since the reaction requires the
loss of the hydroxide ion (a poor leaving group), a little bit of ‘trickery’ is
required in order to convert the hydroxyl moiety into a better leaving group.
This can be achieved under acidic conditions with the use of HCl or HBr. The acid
serves to protonate the hydroxyl moiety as the first step and then a normal SN1
mechanism takes place where water is lost from the molecule to form an
intermediate carbocation. A halide ion then forms a bond to the carbocation
center in the third step.
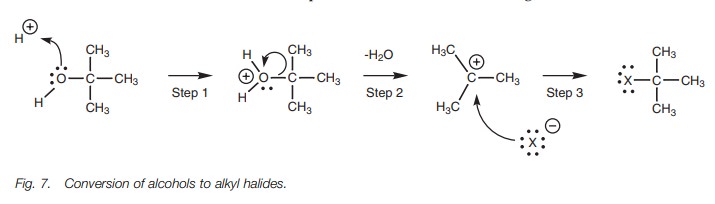
The first two steps of this mechanism are
exactly the same as the elimination reaction described above. Both reactions
are carried out under acidic conditions and one might ask why elimination does
not occur. The difference here is that halide ions serve as good nucleophiles
and are present in high concentration. The elimination reaction described
earlier is carried out using concentrated sulfuric acid and only weak
nucleophiles are present (i.e. water) in low concentration. Having said that,
some elimination can occur and although the reaction of alco-hols with HX
produces mainly alkyl halide, some alkene by-product is usually present.
Since primary alcohols and some secondary
alcohols do not undergo the SN1 reaction, nucleophilic substitution
of these compounds must involve an SN2 mech-anism. Once again,
protonation of the OH group is required as a first step, then the reaction
involves simultaneous attack of the halide ion and loss of water. The reaction
proceeds with good nucleophiles such as the iodide or bromide ion, but fails
with the weaker nucleophilic chloride ion. In this case, a Lewis acid needs to be added to the reaction mixture.
The Lewis acid forms a complex with the oxygen of the alcohol group, resulting
in a much better leaving group for the subsequent SN2 reaction.
Nevertheless, the reaction of primary and
secondary alcohols with hydrogen halides can often be a problem since unwanted
rearrangement reactions often take
To avoid this, alternative procedures carried out under milder basic condi-tions have been used with reagents such as thionyl chloride or phosphorus tribromide (Fig. 8). These reagents act as electrophiles and react with the alcoholic oxygen to form an intermediate where the OH moiety has been converted into a better leaving group. A halide ion is released from the reagent in this process, and this can act as the nucleophile in the subsequent SN2 reaction.
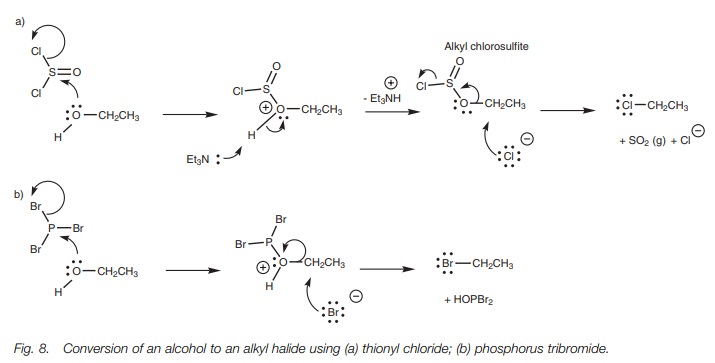
In the reaction with thionyl chloride,
triethylamine is present to mop up the HCl formed during the reaction. The
reaction is also helped by the fact that one of the products (SO2)
is lost as a gas, thus driving the reaction to completion.
Phosphorus tribromide has three bromine
atoms present and eachPBr3molecule can react with three
alcohol molecules to form three molecules of alkyl bromide.
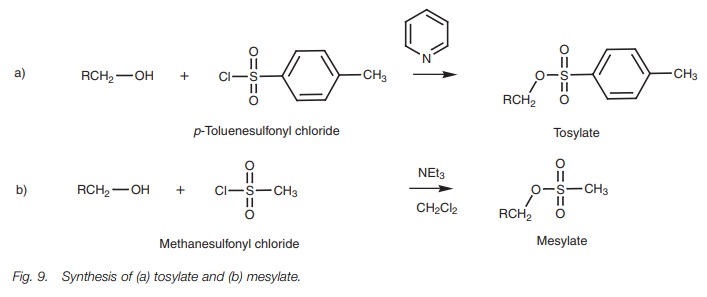
Synthesis of mesylates and tosylates
It is sometimes convenient to synthesize an activated alcohol which can be used in nucleophilic substitution reactions like an alkyl halide. Mesylates and tosylates are examples of sulfonate compounds which serve this purpose.
They are synthesized by treating alcohols with sulfonyl
chlorides in the presence of a base such as pyridine or triethylamine (Fig. 9). The base serves to ‘mop up’ the
HCl which is formed and prevents acid-catalyzed rearrangement reactions.
The reaction mechanism (Fig. 10) involves the alcohol oxygen acting as a nucleo-philic
center and substituting the chloride ion from the sulfonate. The base then
removes a proton from the intermediate to give the sulfonate product. Neither
of these steps affects the stereochemistry of the alcohol carbon and so the
stereo-chemistry of chiral alcohols is retained.
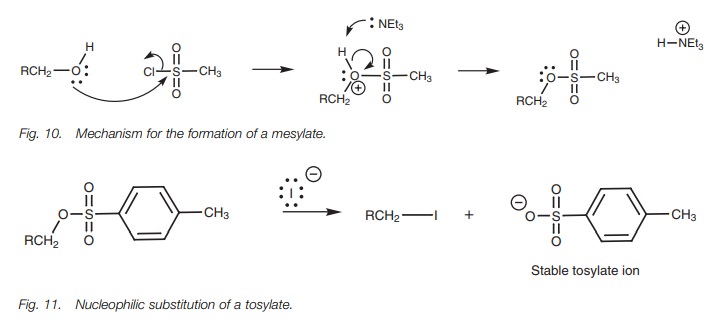
The mesylate and tosylate groups are excellent leaving groups and can be viewed as the equivalent of a halide. Therefore mesylates and tosylates can undergo the SN2 reaction in the same way as alkyl halides (Fig. 11).
Oxidation
The oxidation of alcohols is an extremely
important reaction in organic synthesis. Primary alcohols can be oxidized to
aldehydes, but the reaction is tricky since there is the danger of
over-oxidation to carboxylic acids. With volatile aldehydes, the aldehydes can
be distilled from the reaction solution as they are formed. However, this is
not possible for less volatile aldehydes. This problem can be overcome by using
a mild oxidizing agent called pyridinium chlorochromate (PCC; Fig. 12a). If a
stronger oxidizing agent is used in aqueous conditions (e.g. CrO3 in aqueous sulfuric acid), primary alcohols
are oxidized to carboxylic acids (Fig. 12b), while secondary alcohols are
oxidized to ketones (Fig. 12c).
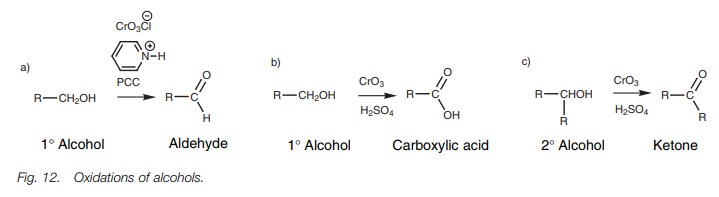
The success of the PCC oxidation in stopping at
the aldehyde stage is solvent dependent. The reaction is carried out in
methylene chloride, whereas oxidation
Under aqueous conditions, the aldehyde which is formed by oxidation of
the alcohol is hydrated and this structure is more sensitive to oxidation than
the aldehyde itself (Fig. 13). In
methylene chloride, hydration cannot occur and the aldehyde is more resistant
to oxidation.
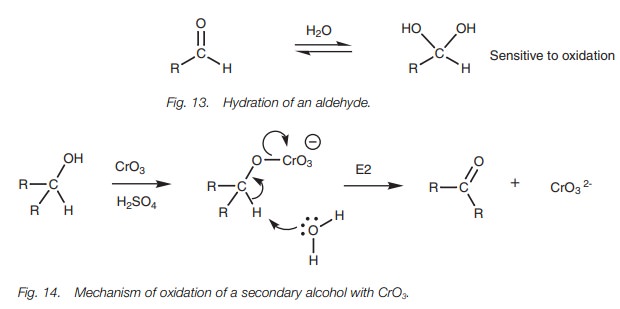
The mechanism of oxidation for a secondary
alcohol with CrO3 (Fig. 14)
involves the nucleophilic oxygen reacting with the oxidizing agent to produce a
charged chromium intermediate. Elimination then occurs where anα-proton is lost along with the chromium moiety to produce the
carbonyl group. The mecha-nism can be viewed as an E2 mechanism, the difference
being that different bonds are being created and broken. Since the mechanism
requires anα-proton to be removed from the alcoholic
carbon, tertiary alcohols cannot be oxidized since they do not contain such a
proton. The mechanism also explains why an aldehyde product is resistant to
further oxidation when methylene chloride is the solvent (i.e. no OH present to
react with the chromium reagent). When aqueous condi-tions are used the
aldehyde is hydrated and this generates two OH groups which are available to
bond to the chromium reagent and result in further oxidation.
Esterification
Alcohols can be converted to esters by
treatment with an acid chloride, acid anhydride or carboxylic acid.
Related Topics