Chapter: Organic Chemistry: Alcohols, phenols, and thiols
Preparation of phenols
PREPARATION OF PHENOLS
Key Notes
Incorporation
A phenol
group can be incorporated into an aromatic ring by sulfonation, followed by
conversion of the sulfonic acid group into the phenol by heat-ing in strong
base. The reaction is limited to alkyl substituted phenols. Alternatively, the
aromatic ring can be nitrated and the nitro group reduced to an aniline, which
is then converted to a diazonium salt and hydrolyzed. Although longer, the
method is more general and a wider variety of substituents is tolerated.
Functional group transformations
Phenyl
esters can be hydrolyzed to their constituent carboxylic acid and phenol. Aryl
ethers are cleaved by heating with HI or HBr to give an alkyl halide and a
phenol.
Incorporation
Phenol groups can be incorporated into an
aromatic ring by sulfonation of the aromatic ring followed by melting the
product with sodium hydroxide to convert the sulfonic acid group to a phenol (Fig. 1). The reaction conditions are
harsh and only alkyl-substituted phenols can be prepared by this method.
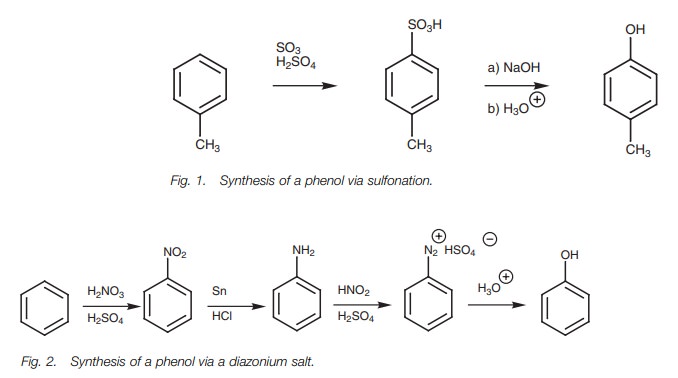
A more general method of synthesizing phenols is to hydrolyze a diazonium salt, prepared from an aniline group (NH2).
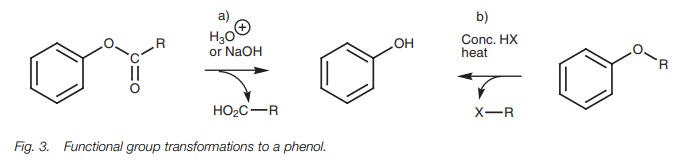
Functional group transformation
Various functional groups can be converted to
phenols. Sulfonic acids and amino groups have already been mentioned. Phenyl
esters can be hydrolyzed. Aryl ethers can be cleaved. The bond between the
alkyl group and oxygen is specifically cleaved since the Ar–OH bond is too
strong to be cleaved.
Related Topics