Chapter: Organic Chemistry: Alcohols, phenols, and thiols
Reactions of phenols
REACTIONS OF PHENOLS
Key Notes
Acid–base reactions
Phenols
are stronger acids than alcohols and are converted to phenoxide ions with
sodium hydroxide. However, they are weaker acids than car-boxylic acids and do
not react with sodium hydrogen carbonate. Electron-withdrawing substituents on
the aromatic ring increase acidity whereas electron-donating groups decrease
acidity. The position of substituents on the aromatic ring relative to the
phenolic group is also important.
Functional group transformations
Phenols
can be treated with acid chlorides or acid anhydrides to give esters. Treatment
with sodium hydroxide then an alkyl halide leads to the forma-tion of aryl alkyl
ethers. There are several reactions which are possible for alcohols but not for
phenols. The synthesis of phenyl esters by reaction with a carboxylic acid
under acid conditions is not possible. Reactions involving the cleavage of the
aryl C–O bond are also not possible.
Electrophilic substitution
Phenols
are powerful activating groups which direct electrophilic substitu-tion to the ortho and para positions. Sulfonation and nitration result in ortho and para products. Bromination, however, results in the introduction of
three bromine substituents at the para
and both ortho positions. The
activat-ing power of the phenol group can be moderated by conversion to an
ester such that bromination occurs only once and is directed para in preference to ortho.
Oxidation
Phenols
are susceptible to oxidation to quinones.
Claisen rearrangement
Phenols
can be converted to phenoxide ions then treated with an allyl bromide to form
an allyl phenyl ether. On heating, these ethers undergo a concerted
rearrangement reaction which results in the allyl group being transferred from
the phenol group to the ortho carbon.
The reaction is a use-ful method of obtaining ortho-alkyl phenols since the double bond can be subsequently
hydrogenated.
Acid–base reactions
Phenols are stronger acids than alcohols and
react with bases such as sodium hydroxide to form phenoxide ions. However, they
are weaker acids than carboxylic acids and do not react with sodium hydrogen
carbonate.
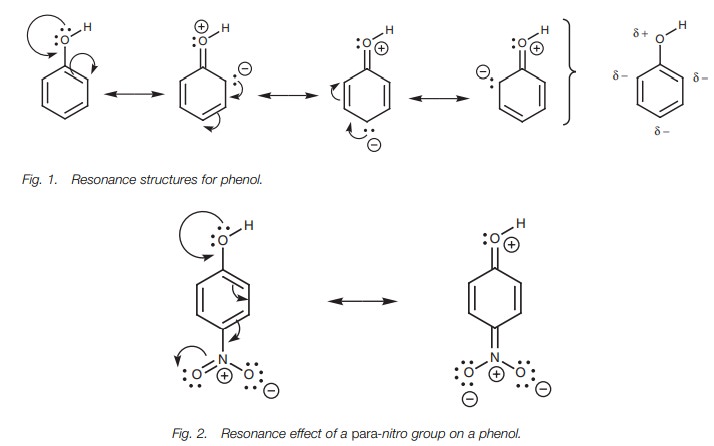
Phenols are acidic since the oxygen’s lone pair of electrons can participate in a resonance mechanism involving the adjacent aromatic ring (Fig. 1). Three resonance structures are possible where the oxygen gains a positive charge and the ring gains a negative charge.
The net
result is a slightly positive charge on the oxygen which accounts for the
acidity of its proton. There are also three aromatic carbons with slightly
negative charges.
The type of substituents present on the aromatic
ring can have a profound effect on the acidity of the phenol. This is because
substituents can either stabilize or destabilize the partial negative charge on
the ring. The better the partial charge is stabilized, the more effective the
resonance will be and the more acidic the phenol will be. Electron-withdrawing
groups such as a nitro substituent increase the acid-ity of the phenol since
they stabilize the negative charge by an inductive effect. Nitro groups which
are ortho or para to the phenolic group have an even greater effect. This is
because a fourth resonance structure is possible which delocal-izes the partial
charge even further (Fig. 2).
Electron-donating substituents (e.g. alkyl
groups) have the opposite effect and decrease the acidity of phenols.
Functional group transformations
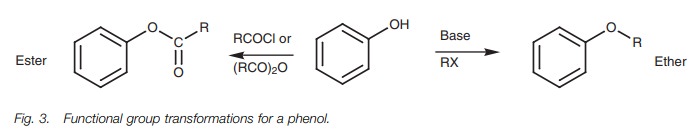
Phenols can be converted into esters by reaction with acid chlorides or acid anhydrides , and into ethers by reaction with alkyl halides in the presence of base. These reactions can be carried out under milder conditions than those used for alcohols due to the greater acidity of phenols. Thus phenols can be converted to phenoxide ions with sodium hydroxide rather than metallic sodium.
Although the above reactions are common to
alcohols and phenols, there are several reactions which can be carried out on
alcohols but not phenols, and viceversa.
For example, unlike alcohols, phenols cannot be converted to esters by
reac-tion with a carboxylic acid under acid catalysis. Reactions involving the
cleavage of the C–O bond are also not possible for phenols. The aryl C–O bond
is stronger than the alkyl C–O bond of an alcohol.
Electrophilic substitution
Electrophilic
substitution is promoted
by the phenol
group which acts as an
activating group and directs substitution to the ortho and para positions.
Sulfonation and nitration of phenols are both possible to give ortho and para sub-stitution products. On occasions, the phenolic groups may
be too powerful an activating group and it is difficult to control the reaction
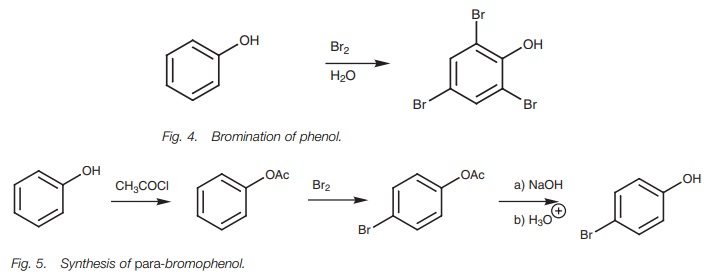
to one substitution. For example, the bromination of phenol leads to
2,4,6-tribromophenol even in the absence of a Lewis acid (Fig. 4).
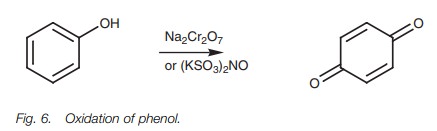
The activating power of the phenolic group can be decreased by converting the phenol to an ester which can be removed by hydrolysis once the electrophilic sub-stitution reaction has been carried out (Fig. 5). Since the ester is a weaker activat-ing group, substitution occurs only once. Furthermore, since the ester is a bulkier group than the phenol, para substitution is favored over ortho substitution.
Oxidation
Phenols are susceptible to oxidation to
quinones (Fig. 6).
Claisen rearrangement
A useful method of introducing an alkyl
substituent to the ortho position of
a phenol is by the Claisen rearrangement (Fig.
7). The phenol is converted to the phenoxide ion, then treated with
3-bromopropene (an allyl bromide) to form an ether. On heating, the allyl group
(–CH2–CH=CH2) is transferred from the phe-nolic group to
the ortho position of the aromatic
ring. The mechanism involves a
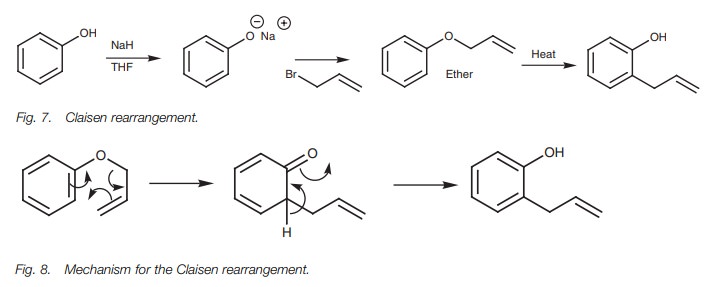
This results in a ketone
structure which immediately tautomerizes tothe final product. Different allylic
reagents could be used in the reaction and the double bond in the final product
could be reduced to form an alkane substituent without affecting the aromatic
ring.
Related Topics