Chapter: Pharmaceutical Drug Analysis: Theory and Technique of Quantitative Analysis
Automated Methods of Clinical Analysis
AUTOMATED METHODS OF CLINICAL ANALYSIS
Theory : An ‘Autoanalyzer’ serves as the most versatile and important instrument
in a well-equipped ‘clinical laboratory’ that caters for the
rapid screening of serum levels for upto forty (40) important chemical
substances in the field of diagnostic medicine. These autoanalyzers may be
either ‘Single Channel’ i.e.,
per-forming only one determination on each sample or Multichannel’ i.e., carrying out several different
determinations on each sample.
A few important substances that are routinely analyzed in
a clinical laboratory with the aid of an ‘Autoanalyzer’ are, namely :
serum-glutamic-oxaloacetic transaminase (SGOT) ; creatine-phophokinase (CPK);
alkaline-phosphatase (AP) belonging to the class of enzymes ; and a host of
biochemical substances, for instance : bilirubin, serum albumin, blood urea
nitrogen (BUN), uric acid, creatinine, total protein, glucose, cholesterol,
besides a few common inorganic ions, such as : Cl–, Ca2+,
K+, Na+.
The basic principles
underlying both automated and unautomated methods of analysis are more or less
the same. Out of the broad-spectrum of biological samples blood analysis is the
most common one. There exists a number of parameters which may be assayed, and
spectrophotometry is ideally suited for nearly all of them, a few typical
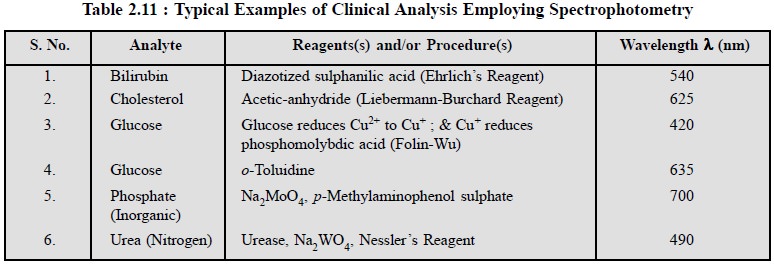
examples are cited in Table 2.11.
Explanation : Glucose (having an aldehyde
functional moiety) reduces Cu2+
to Cu2O (i.e., Cu+)
as per the following reaction :

As some other sugars are also present in blood sample,
and besides the above reaction not being abso-lutely stoichiometric, it has
become necessary in actual practice to establish an emperical calibration curve
using known concentrations of glucose. The above reaction is allowed to proceed
for exactly 8 minutes at 100°C. To the resulting solution phosphomolybdic acid
is added, which is subsequently reduced by Cu2O to give rise to an
intensely coloured ‘molybdenum blue’ that is measured at 420 nm accurately.
Alternatively, glucose forms a
specific complex with o-toluidine
according to the following reaction that forms the basis of the colorimetric
assay :
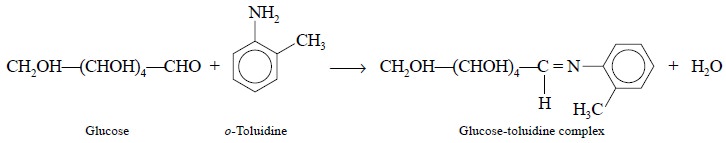
The diagnostic green colour is usually developed for
exactly 10 minutes at 100°C and measured subse-quently at 635 nm.
1. Instrumentation
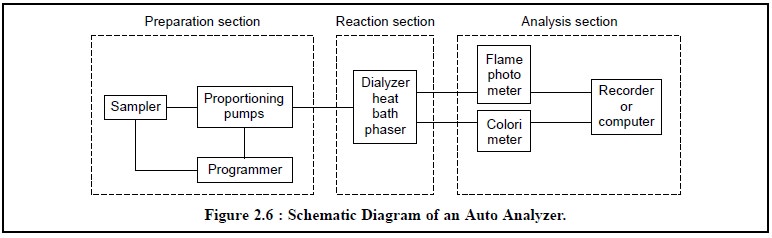
The schematic diagram of an
Auto Analyser is shown in Figure 2.6. The major component parts com-prise of
the various important sections namely : the preparation section, the reaction
section and the analysis section which will be discussed briefly here.
1.1. Preparation Section
This particular section of the Auto Analyzer consists
mainly of the sampler, proportioning pumps, and programmer. First, the sampler
introduces a fixed quantity of serum sample into the ‘analysis train’, which
varies from one instrument to another instrument supplied by different
manufacturers. For instance, the SMA-12 Survey Auto Analyzer possesses 12
analysis trains or streams as illustrated in Figure 2.7.
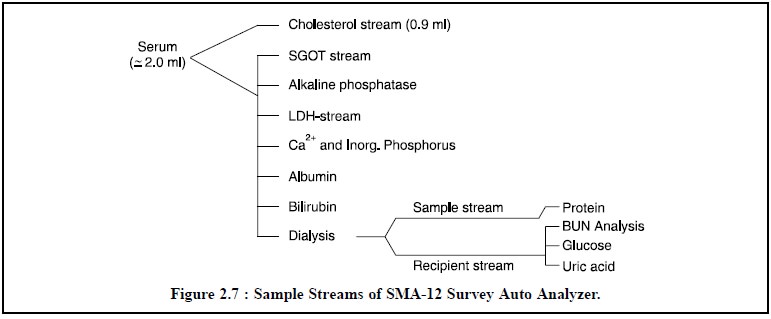
The proportioning pump controls the rate of advancement, viz 10 inch/minute, of each sample through the analysis stream. Hence, a fixed length of tubing is equivalent to a fixed amount of time. Each analysis stream is made of transparent plastic flexible tubing, and each patient-sample is separated from one another by an air-bubble.
1.2. Reaction Section
The reaction section
essentially comprises of the dialyzer, heat bath and phaser, and obviously the
reaction takes place in this zone. Let us consider the following generalized
reaction :

Where , [C]c
= Molar concentration of substance C raised to the cth power,
A = Component in serum (e.g.,
cholesterol), and
B = Reactant that reacts with A to give a coloured product.
Evidently, B is added always
in excess to ensure :
(a)
rapid reaction, and
(b) complete
reaction by forcing the reaction to the right in accordance to the Le Chatelier’s principle.
Now, the rate of forward reaction = k [A]a [B]b
Hence, the rate constant may be expressed as follows :
k = Ae–Ea/RT ...............(c)
where , R = Gas constant ( 1.99
cal/K-mol),
T = Temperature, and
Ea =
Activation energy of the reaction as depicted in Figure 2.8.
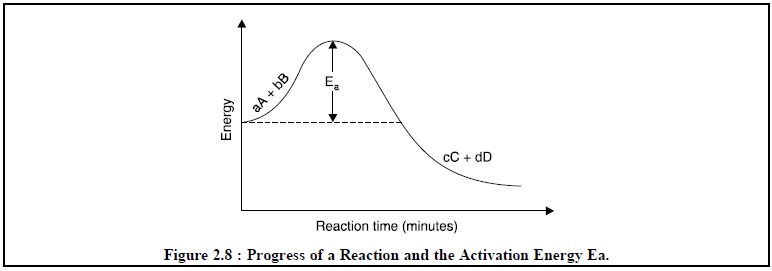
From Eq. (c) it
may observed that as the temperature T is enhanced then the rate of reaction
also enhances simultaneously because a higher value of T offers a smaller
negative exponent of e or a larger
number. Therefore, in actual experimental operations temperature is increased
by the aid of a heat-bath so as to accelerate the reaction which in turn allows
the reaction to attain equilibrium state as rapidly as possible.
Naturally at a very high temperature there is every
possibility for decomposition of either the products or the reactants.
1.3. Analysis Section
The recent advancement in the field of computer technology and anlytical instrumentation it has become
very easy and convenient to have the analyical data from a series of biological
samples processed at high speed as digital readouts or on computerized
recorders. Many hospitals round the globe make extensive use of advanced
computer softwares for data processing as stated beiow :
·
Uptodate listing of various laboratory tests,
·
Listing of drugs and metabolites that cause interference
both biochemically and analytically,
·
Storing of levels of biologically important compounds for
various disease states, and
·
A tentative diagnosis for a patient based on his serum
sample under investiation together with the drugs and dosages being
administered and the levels of biologically important compounds.
Caution : Nevertheless, the concerned
physician or pharmacist must exercise his or her own expertise and knowledge while prescribing
drug(s) to a patient along with these computerized data informations.
Related Topics