Chapter: Genetics and Molecular Biology: Oncogenesis, Molecular Aspects
ras-fos-jun Pathway
The ras-fos-jun
Pathway
The ras
protein is one well-studied oncogene, partially because it is encoded by the oncogene
Weinberg found in the transformation assays of the NIH 3T3 cells. More
extensive studies have shown that an activated ras product is found in 10 to 20% of all human cancers. Humans
possess three different ras products,
N-ras, which is encoded on chromosome
1, H-ras from chromosome 11, and K-ras from chro-mosome 12. Even Saccharomyces cerevisiae possesses two ras products.
In order that these ras proteins be able to respond
to extracellular and transmembrane receptors, they are held on the inner surface
of the plasma membrane by farnesyl. This is a fatty acid, membrane-loving,
intermediate of the cholesterol biosynthetic pathway. The farnesyl is attached
to the carboxy terminal cysteine of ras. The same principle of attaching a
membrane-soluble molecule to a protein is used to attach the outer membrane of Escherichia coli to the peptidoglycan
layer. In yeast, the same enzymes that transfer the fatty acid to the cysteine
of ras for membrane attachment also modify a
mating-type factor. Al-though ras proteins bind nucleotides and have a GTPase
activity and are therefore G proteins, they function as monomers, whereas many
other G proteins function as heterotrimers.
The G proteins are activated by the binding of a
GTP nucleotide. When they hydrolyze this to GDP, the activation ceases. Not
surpris-ingly, then, oncogenic ras
mutants do not hydrolyze the GTP normally. Some have mutations in the
GTP-binding site, and others have lost the ability to bind to an accessory
protein, GAP, for GTPase activating protein (Fig. 23.12). It is the GAP protein
that interacts with the growth factor receptor. When the receptor is occupied,
the GAP protein is phosphorylated. It therefore ceases to stimulate hydrolysis
of GTP bound to ras. This leaves ras in an activated state. As a result, it
activates a phosphorylation pathway that ultimately phosphorylates and
acti-vates the c-jun protein.
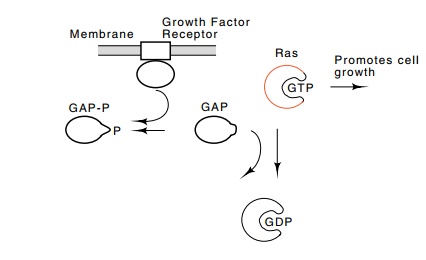
Figure
23.12 Pathway for activation of ras
protein
Phosphorylated c-jun then forms a heterodimer with
c-fos protein. This heterodimer is also known as AP-1 and it activates
transcription from a wide collection of promoters.
The fos
and jun oncogenes were identified on
retroviruses and the cellular analogs were then found using their homology to
the viral forms. These proteins are typical leucine zipper transcriptional
activa-tors and they bind to the same DNA sequence as the yeast general amino
acid regulator protein GCN4. The structure of the fos and jun leucine zippers
favors a heterodimer rather than homodimer formation due to the presence of
opposite charges on the two helices. The theme of heterodimer formation
regulating cell growth is common and both leucine zipper proteins and
helix-loop-helix proteins participate in this form of regulation.
Related Topics