Chapter: Genetics and Molecular Biology: Oncogenesis, Molecular Aspects
Identification of the src and sis Gene Products
Identification of the src and sis Gene Products
Eukaryotic cells contain thousands of proteins.
Most likely, only a few of these are involved in regulation of growth, and
these probably are synthesized in small quantities. Therefore it would seem
like a difficult task to identify a protein as the product of either c-src or v-src and virtually impossible to determine the enzymatic activity
of such a protein. Nonetheless, Erikson accomplished both for the v-src
protein.
The starting point of this work was the fact that animals with sarcomas or lymphomas frequently synthesize antibodies against the retroviral proteins. The v-src protein might also induce antibody syn-thesis in animals if it were sufficiently different in structure from all other cellular proteins. Therefore rabbits were infected with an avian sarcoma virus that could induce sarcomas even in some other types of animals. The same virus was also used to transform chick embryo fibroblasts in vitro. These chicken cells ought to synthesize the same viral proteins as the rabbit. As a result, these proteins would be recognized by the rabbit antibodies. In addition, the chicken embryo fi-broblasts ought to contain very few of the other proteins that would be recognized by the rabbit antibodies. Therefore, the v-src product ought to be one of the very few proteins recognized by the antibodies.
Extracts from radioactively labeled RSV-transformed
chick cells were incubated with the serum from the RSV-infected rabbits. Since
the quantities of proteins bound by the antibodies were low, an
antigen-an-tibody lattice was unlikely to form. Another method had to be used
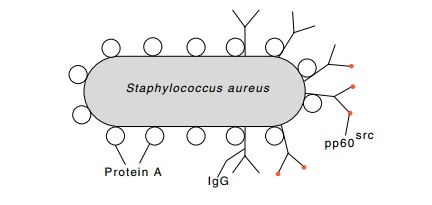
for selectively isolating the antigen bound to antibody. Erikson used whole
cells of Staphylococcus aureus
because as mentioned earlier, one of its surface proteins, protein A,
specifically binds to IgG. After incubation of rabbit serum with the
radioactive chick proteins, the Staphylococcus
cells were added and the mixture centrifuged to separate antibody-bound
radioactive proteins from those not bound to antibody.
About 20 radioactive proteins from the chick cells
were precipitated by the rabbit serum. Among them was one that was present only
if the labeled proteins originated from transformed chick cells. Furthermore,
this protein was not present if the cells had been infected with a nonacutely
transforming retrovirus. In addition, the protein was the same molecular weight
as a protein synthesized in vitro
using the oncogene portion of RSV as a messenger. In this way, the v-src gene product was identified as a
60,000 molecular weight protein, which is called p6Osrc.
An enzymatic activity associated with src was discovered on the basis of an educated guess. Since the protein probably is involved with regulating growth and since phosphorylation of proteins is involved in regulating many activities in eukaryotic cells, the src protein could be a protein kinase. Therefore radioactive ATP and potential kinase sub-strate proteins were included with the Staphylococcus-precipitated IgG-bound src protein. Indeed, a kinase activity was detected. It was shown to be the product of the src gene because it was absent from cells grown at high temperature that had been transformed with mutants of RSV that do not transform at high temperature. While most amino acid-linked phosphate in cells is phosphothreonine or phosphoserine, less than 0.03% is phosphotyrosine. Following RSV infection, the levels of phosphotyrosine dramatically increase. These elevated levels fall if the infecting virus possess a temperature-sensitive kinase and the temperature of the cell culture is raised. Together, these pieces of evidence provide solid proof that the kinase is the product of v-src.
Another related question is whether the antibodies
against the v-src gene product could
detect the c-src gene product. They
could, even though the protein is synthesized at a small fraction of the level
at which the viral homolog is synthesized. The cellular protein also is a
kinase. Similar studies have permitted partial purification of a number of
other viral and cellular oncogene products. In many cases the cellular
loca-tions of these proteins can also be determined by fractionation of
cellular components or with labeled antibodies.
The actual cellular function of the oncogene
carried on a simian retrovirus, v-sis,
has also been identified. This was not done via antibod-ies and enzyme assays.
Instead, the nucleotide sequence of its oncogene identified its product as the
platelet-derived growth factor. This had been purified for entirely different
reasons from blood plasma. It is released by platelets at the sites of wounds
and stimulates growth of fibroblasts, smooth muscle cells, and glial cells.
When the amino acid sequence for the growth factor was entered into a data base
and homologies were sought, the simian virus oncogene immediately was revealed
as encoding a close relative. Almost certainly the retrovirus gene derives from
the growth factor gene.
Related Topics