Chapter: Modern Pharmacology with Clinical Applications: Hypocholesterolemic Drugs and Coronary Heart Disease
When to Treat Hypertriglyceridemias
When to Treat
Hypertriglyceridemias
The guidelines for use of
drugs to treat familial hyper-triglyceridemia type IV are less well defined
than those for hypercholesterolemia. One should account for plasma HDL in
deciding to treat hypertriglyceridemias with the intent of decreasing the risk
for CHD. Moderate hypertriglyceridemia (200–500 mg/dL) with-out low HDL may not
be an independent risk factor for CHD. However, the results of a recent
clinical trial indi-cate that hypertriglyceridemia is an independent risk
factor for ischemic stroke. Results of the Helsinki Heart Study showed that the
reduced risk of CHD with use of gemfibrozil (discussed later) was correlated
with eleva-tion of HDL plus reduction of VLDL triglyceride rather than
reduction of LDL cholesterol. Gemfibrozil has lit-tle effect on plasma LDL.
Low HDL cholesterol ( 35
mg/dL) is an independ-ent risk factor for CHD. HDL appears to antagonize
atherogenesis by at least two mechanisms. HDL can mobilize cholesterol from
extrahepatic cells (such as ar-terial wall foam cells) and transport it to the
liver for disposal (reverse cholesterol transport); HDL also has antioxidant
properties. HDL contains the potent an-tioxidant enzyme paraoxonase, which may
protect LDL lipids from oxidation. Thus, hypertriglyceridemia with concurrent
low HDL cholesterol should be treated to reduce the risk of CHD. Treatment of
hypertriglyc-eridemia independent of HDL levels may also be worthwhile to
decrease the risk of ischemic cerebrovas-cular disease. Very high plasma
triglycerides ( 1000 mg/dL) are clearly a risk factor for pancreatitis and must
be treated for this reason.
As with drugs that lower LDL
cholesterol, dietary plus other lifestyle changes should accompany drug therapy
of hypertriglyceridemia. Reduction of body weight to ideal is probably the
single most important di-etary goal. Because patients with familial
hypertriglyc-eridemia may have increased liver capacity to synthe-size fat from
carbohydrate, attention should be given to restricting excessive carbohydrate
and alcohol.
Fibrates
Mechanism of Action
The three structurally
related fibrates available in the United States are gemfibrozil (Lopid), fenofibrate (Tricor) and clofibrate (Atromid-S). They share common uses and
toxicities. The fibrates typically lower VLDL triglyceride by 40% or more and
elevate plasma HDL cholesterol by 10 to 15%. The reduction of plasma
triglycerides in humans appears due to increased lipopro-tein lipase (LPL)
activity. The fibrates activate a nuclear receptor (transcription factor)
termed peroxisomal pro-liferation activated receptor (PPAR) that is a member of
the steroid hormone receptor superfamily. PPAR in-creases transcription of the
LPL gene and decreases tran-scription of the apolipoprotein CIII gene (apo
CIII). Since LPL is responsible for catabolism of VLDL triglyc-eride and apo
CIII is an inhibitor of LPL activity, the combined consequences of these
changes are increased LPL activity and enhanced removal of triglyceride from
the circulation (mechanism II in Fig. 23.2).

The elevation of HDL levels
by fibrates may be due to two drug actions: induced synthesis of apo-A1, the
principal apoprotein of HDL, and increased assembly of new HDL particles in the
circulation. Surface com-ponents of VLDL contribute to formation of HDL, as the
VLDL particles are reduced in size through the ac-tion of LPL. The increased
rate of catabolism of VLDL caused by the fibrates would provide more components
for assembly of HDL particles.
Clinical Uses
The fibrates are mainly used
to treat two hyperlipi-demias, familial hypertriglyceridemia (type IV) and
dysbetalipoproteinemia (type III). They are also useful in the treatment of
hypertriglyceridemia associated with type II diabetes (secondary
hyperlipidemia). The fibrates are the drugs of choice in treating
hypertriglyc-eridemias, particularly those associated with low levels of HDL
cholesterol. The fibrates additionally appear to shift LDL particles to larger,
hence less atherogenic, species.
Type III or
dysbetalipoproteinemia is a rare condi-tion in which cholesterol-enriched VLDL
remnants, called -VLDL, accumulate in the plasma. They are atherogenic
particles. Dysbetalipoproteinemia is a ge-netic condition associated with expression
of an unusual form of apolipoprotein E (apo E2 versus the normal E3) that leads
to reduced plasma clearance of these lipoproteins by the liver. Through
stimulation of LPL and perhaps other lipases, the fibrates accelerate
clear-ance of these -lipoproteins. Both plasma cholesterol and triglyceride
levels are elevated in dysbetalipopro-teinemia and in combined hyperlipidemia,
type IIb. However, the drug treatments are different for the two conditions.
Type IIb hyperlipoproteinemia requires use of agents that lower both LDL and
VLDL particles; for example, a statin plus niacin, niacin alone, or niacin in
combination with a fibrate. Care should be taken in dis-tinguishing between
types IIb and III as the cause of the elevated cholesterol plus triglyceride.
This can be achieved by examining the profile of the elevated plasma
lipoproteins separated by electrophoresis. A broad -band is seen in type III
but distinct - and pre--bands are seen in type IIb.
Adverse Effects
The fibrates are generally
well tolerated, with GI distress being the most likely complaint. Other adverse
effects include myositis and erectile dysfunction, partic-ularly with
clofibrate. There is ongoing concern about the fibrates increasing the risk of
gallstones, although the extent of risk is unclear. Because clofibrate was
as-sociated with increased mortality in early clinical trials, it should be
considered as a second-line drug.
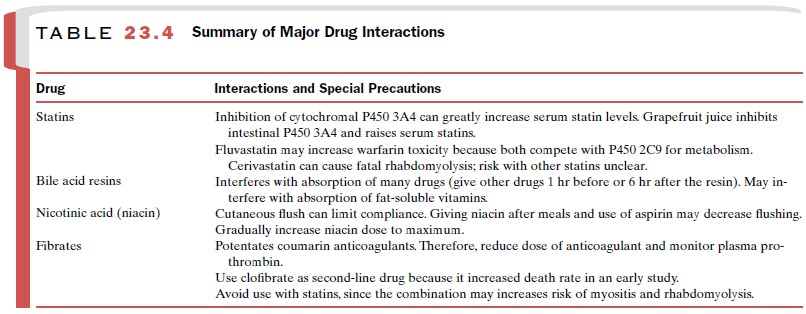
Drug Interactions
The fibrates potentiate the
actions of the coumarin anticoagulants, such as warfarin, so care should be
taken to reduce the dose of simultaneously administered anti-coagulants, and
plasma prothrombin should be fre-quently measured until the level stabilizes.
As men-tioned earlier, great care should be given to combining a statin with a
fibrate, since this combination may in-crease the risk of myositis and perhaps
rhabdomyolysis. Table 23.4 summarizes major interactions of drugs that lower
cholesterol.
Related Topics