Chapter: Clinical Cases in Anesthesia : Spinal Anesthesia
What are the recognized complications of spinal anesthesia?
What are the recognized
complications of spinal anesthesia?
The single most common complication of spinal
anesthesia is probably hypotension. Postganglionic autonomic nerves, which are
small, unmyelinated C fibers, are exquisitely sensitive to spinal blockade.
The greater the extent of anesthesia, the greater the sympathectomy.
Interruption of sympathetic stimuli to the capacitance vessels markedly
increases peripheral venous pooling, resulting in decreased venous return to
the heart. Consequently, cardiac output falls. The usual compensatory response
to reduced cardiac output is an increase in heart rate. Sudden tachycardic
responses are mediated through the cardiac accelerator nerves, which receives
contributions from spinal nerves T1–T4. Blockade of the
upper thoracic nerve roots not only prevents acute increases in heart but also
allows for unopposed vagal influence, thereby slowing heart rate. Therefore,
cardiac output is impaired by two mechanisms: peripheral venous pooling and
bradycardia.
Before administration of spinal anesthesia,
fluid loading with approximately 500 mL of a balanced salt solution helps to
prevent the state of relative hypovolemia induced by venous dilatation. Most
cases of spinal-induced hypotension respond favorably to altering the patient’s
position into 10° of Trendelenburg, lithotomy, or left lateral
uterine displacement. Intravenous volume infusion is frequently required to
restore blood pressure toward its normal range. Hypotension unresponsive to
fluid adminis-tration requires immediate treatment and usually responds to
ephedrine, 5–10 mg, intravenously. Ephedrine works by stimulating both α- and β-receptors causing an increase in the heart
rate, contractility, and peripheral resistance, which are frequently sufficient
to correct hypotension. Hypotension, dysrhythmias, and myocardial ischemia are
associated untoward effects. Ephedrine causes little or no alteration in
uterine blood flow. Alternatively, a continuous infusion of phenylephrine may
return vascular tone toward normal. The solution is frequently prepared by
adding 10 mg of phenylephrine to 250 or 500 mL of 5% dextrose in water.
Phenylephrine acts as an α-adrenergic agonist. Its side-effects include
hypertension, bradycardia, and uterine vasoconstriction. It is not the first
choice for treating hypotension in the pregnant patient. Bradycardia is
effec-tively treated with atropine 0.4 mg intravenously, or ephedrine in small
doses.
Ventilatory impairment frequently results from
hypotension leading to impaired medullary blood flow and hypoxia of the
respiratory center. Blockade of the phrenic nerve, composed of contributions
from C3–C5, leading to impaired diaphragmatic movement,
is highly unusual. Motor blockade of the intercostal and abdominal muscles may
prevent effective coughing. Loss of the intercostal muscle’s proprioception
frequently prevents the patient from appreciating chest expansion, thereby creating
a sub-jective feeling of difficulty breathing.
Nausea and vomiting accompanying spinal
anesthesia often result from parasympathetic imbalance, hypoten-sion, or
hypoxemia. Treatment with atropine, vasopressors, or oxygen usually provides
relief. Retching, apprehension, agitation, and shortness of breath may also be
secondary to hypotension or hypoxemia. Treatment requires increasing the blood
pressure, oxygen administration, and assisted or controlled ventilation.
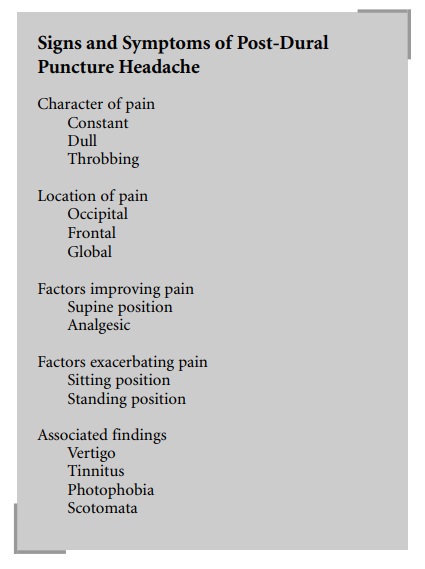
Post-dural puncture headache (PDPH) remains the
most commonly encountered postanesthetic side-effect of spinal anesthesia. The
frequency of PDPH following dural puncture with a 17-gauge Tuohy needle has
been reported to be as high as 75%. The incidence of PDPH is lower in the
elderly and in those whose dura is punctured by a pencil-point or a fine
needle. PDPH following the use of a 26-gauge needle may be as low as 2.5%. In
an ambulatory setting, Kang et al. (1992) noted a PDPH rate of 9.6% and 1.5%
associated with 26- and 27-gauge needles, respec-tively. Aligning the needle
bevel parallel to the dural fibers seems to markedly reduce the incidence of
PDPH. This approach tends to separate rather than cut the longitudinal dural
fibers, resulting in a smaller, more readily repairable hole.
PDPH following subarachnoid block emanates from
traction on the meninges and vascular structures, as CSF leaks through the
dura. Symptomatic treatment requires mild analgesics, bed rest, and fluid
administration. Injection of morphine into the subarachnoid space along with a
local anesthetic does not decrease the incidence of PDPH. In patients with
severe incapacitating headaches, or headaches of several days’ duration, an
epidural blood patch is indicated. An epidural blood patch is performed by
placing a needle in the epidural space at the suspected level of dural
puncture. Fifteen to twenty milliliters of the patient’s own blood, drawn under
sterile conditions, are injected through the newly placed epidural needle. This
maneuver is highly successful, but risk of re-puncturing the dura exists.
Slight elevations in temperature are occasionally seen for 1 or 2 days
following this procedure. Low back pain and neck discomfort have also been
reported following epidural blood patching. Caffeine, a cerebral vasoconstrictor,
may also provide beneficial effects. Other causes of PDPH, such as septic or
aseptic meningitis and arachnoiditis, are extremely rare. Urinary retention
that is due to prolonged blockade has also been associated with spinal
techniques.
Backache occurs frequently following spinal
anesthesia but is usually short-lived and of only mild-to-moderate intensity.
Its causes generally include lumbar ligamentum strain, paraspinous muscle
spasm, and muscle hematoma formation. Severe back pain requires immediate
neurologic investigation.
The incidence of major neurologic sequelae
following spinal anesthesia approaches 0.5%. If exacerbations of pre-existing
neurologic diseases are eliminated from this figure, the probability of
encountering neurologic damage following spinal anesthesia diminishes even
further. Transient radicular irritation (TRI) consists of pain, and/or
dysesthesia in the legs or buttocks. This occurred more frequently with
lidocaine, but has been seen with tetracaine and bupivacaine. Other factors
that contribute to the incidence of TRI are the lithotomy position, ambu-latory
patients, and obesity. TRI usually resolves within 72 hours but may take as
long as 6 months.
Hematoma or abscess formation producing a cauda
equina syndrome is potentially identifiable and reme-diable. Numerous cases of
spontaneous subarachnoid and epidural hemorrhage exist in anticoagulated
patients. Current American Society of Regional Anesthesia and Pain Medicine
(ASRA) recommendations concerning the use of spinal or epidural anesthesia in
patients on antiplatelet drugs are as follows. Nonsteroidal anti-inflammatory
drugs and aspirin do not present an increased risk for intraspinal bleeding
when used as a single agent. Use of spinal or epidural anesthesia is at the
discretion of the anesthesiologist. However, there is the known risk of a
hemorrhagic complication when these drugs are concur-rently given with other
antiplatelet drugs such as heparin, low-molecular-weight heparin, warfarin,
ticlopidine (Ticlid), or clopidogrel (Plavix). If spinal or epidural anesthesia
is considered, there should be careful documentation of the lack of therapeutic
effect (normal coagulation tests) of the second drug. Regarding the new
antiplatelet drugs, ticlopidine and clopidogrel, which are drugs prescribed for
prevention of myocardial infarction, stroke, and vaso-occlusive disorders,
there are no current studies to establish the safety of performing regional
anesthesia during their use.
There are also no data regarding their
interaction with other anticoagulant drugs. Thus, ASRA guidelines recommend
dis-continuing ticlopidine for 10–14 days and clopidogrel for 7 days prior to
performing a spinal or epidural anesthetic.
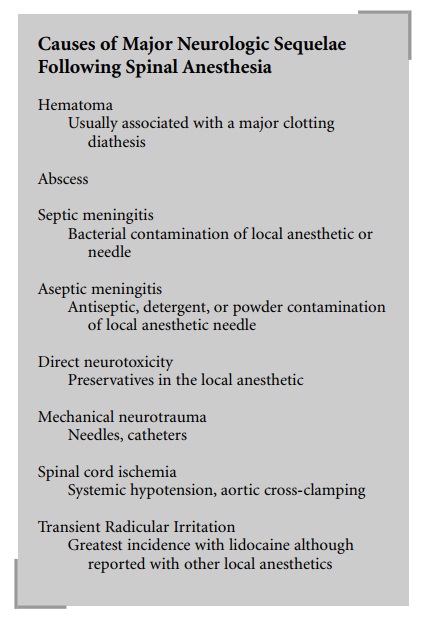
Direct neurotoxicity of commonly used local
anesthetic solutions is almost nonexistent. Chloroprocaine, however, represents
a notable exception. Although apparently free of direct neurotoxicity in the
epidural space, chloroprocaine possesses neurolytic properties in the
subarachnoid space. Sodium bisulfite, a preservative, has been identified as
the causative agent. Sodium bisulfite has been eliminated from many currently
available preparations. At present, chloro-procaine is not recommended for use
in the subarachnoid space. Cauda equina syndrome has been reported follow-ing
administration of hyperbaric local anesthetics through subarachnoid catheters.
Septic and aseptic meningitis has been attributed to contamination of drugs and
needles with bacteria, detergents, and powder. Single-use, dispos-able
equipment has almost eliminated this problem. Direct neural trauma may
theoretically occur from needles or catheters but should be relegated to a
practical improbabil-ity in most cases. Spinal cord ischemia has been
associated with systemic hypotension and cross-clamping of the aorta.
Pre-existing neurologic disease, improper patient posi-tioning, or pressure
from retractors on the fetal head may also predispose the patient to neurologic
defects following spinal anesthesia and are unrelated to the spinal anesthetic.
Failure of spinal anesthesia to provide
adequate analge-sia remains another commonly encountered complication.
Prospective studies have estimated the rate of failed spinals to be 4–16%. A
study by Munhall et al. (1988) demon-strated that only 25% of spinal failures
were due to factors such as inability to identify the subarachnoid space and
lack of free-flowing CSF before, as well as after, injection of local
anesthetic. Most inadequate spinal anesthetics were due to faulty selection of
local anesthetic, dose, vasocon-strictor, baricity, position, interspace, or
single-injection versus catheter technique. An example of such a judgment error
is the selection of tetracaine 0.5% over bupivacaine 0.5% to block tourniquet
pain.
Long-term follow-up studies of patients
receiving large numbers of spinal anesthetics have shown spinal anesthe-sia to
be a safe technique (e.g., Vandam and Dripps 1960).
Related Topics