Chapter: Basic & Clinical Pharmacology : Agents That Affect Bone Mineral Homeostasis
Vitamin D - Principal Hormonal Regulators of Bone Mineral Homeostasis
VITAMIN D
Vitamin D is a
secosteroid produced in the skin from 7-dehydrocholesterol under the influence
of ultraviolet radiation. Vitamin D is also found in certain foods and is used
to supplement dairy products. Both the natural form (vitamin D3, cholecalciferol) and
the plant-derived form (vitamin D2, ergocalciferol) are present in the diet. These forms differ in
that ergocalciferol contains a double bond (C22–23) and an additional methyl group in the side
chain (Figure 42–3). Ergocalciferol and its metabolites bind less well than
cholecalciferol and its metabolites to vitamin D-binding protein, the major
transport protein of these compounds in blood, and have a different path of
catabolism. As a result their half-lives are shorter than those of the
cholecalciferol metabolites. This influ-ences treatment strategies, as will be
discussed. However, the key steps in metabolism and biologic activities of the
active metabolites are comparable, so with this exception the following comments
apply equally well to both forms of vitamin D.
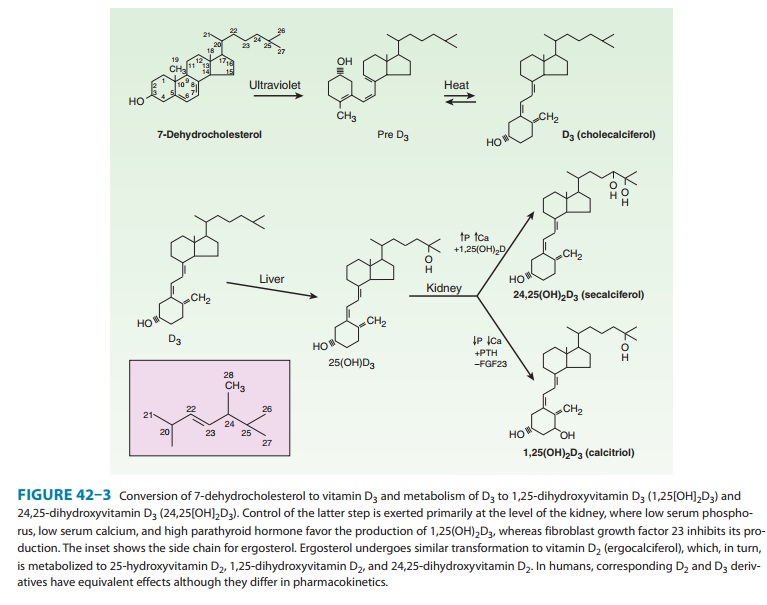
Vitamin D is a precursor to a number of
biologically active metabolites (Figure 42–3). Vitamin D is first hydroxylated
in the liver to form 25-hydroxyvitamin D (25[OH]D, calcifediol). This
metabolite is further converted in the kidney to a number of other forms,the
best studied of which are 1,25-dihydroxyvitamin (1,25[OH] 2D, calcitriol)
and 24,25-dihydroxyvitamin
(24,25[OH]2D).
The regulation of vitamin D metabolism is complex, involving calcium, phosphate,
and a variety of hormones,the most important of which is PTH, which stimulates,
and FGF23, which inhibits the production of 1,25(OH)2D by the
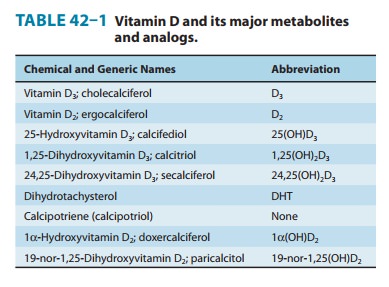
kidney. Of the natural metabolites, only vitamin D and 1,25(OH)2D
(as calcitriol) are available for clinical use (Table 42–1). A number of
analogs of 1,25(OH)2D have been synthesized to extend the usefulness
of this metabolite to a variety of nonclassic conditions. Calcipotriene
(calcipotriol), for example, is being used to treat psoriasis, a
hyper-proliferative skin disorder. Doxercalciferol and parical-citol are
approved for the treatment of secondary hyperparathyroidism in patients with
chronic kidney disease. Other analogs are being investigated for the treatment
of various malignancies.
Vitamin D and its metabolites
circulate in plasma tightly bound to the vitamin D-binding protein. This α-globulin binds 25(OH)D and 24,25(OH)2D with
comparable high affinity and vitamin D and 1,25(OH)2D with lower
affinity. As noted above the affinity for the D2 metabolites is less
than that for the D3
In normal subjects, the terminal half-life of injected calcifediol is 23 days,
whereas in anephric subjects it is 42 days. The half-life of 24,25(OH)2D
is probably similar. Tracer studies with vitamin D have shown a rapid clearance
from the blood. The liver appears to be the principal organ for clearance.
Excess vitamin D is stored in adipose tissue. The metabolic clearance of
calcitriol in humans indicates a rapid turnover, with a terminal half-life
measured in hours. Several of the 1,25(OH)2D
analogs are bound poorly by the vitamin D-binding protein. As a result, their
clearance is very rapid, with a terminal half-life of minutes. Such analogs
have less of the hypercalcemic, hypercalciuric effects of calcitriol, an
important aspect of their use for the management of conditions such as
psoriasis and hyperparathyroidism.
The
mechanism of action of the vitamin D metabolites remains under active
investigation. However, 1,25(OH)2D
is well estab-lished as the most potent agent with respect to stimulation of
intes-tinal calcium and phosphate transport and bone resorption. 1,25(OH)2D
appears to act on the intestine both by induction of new protein synthesis (eg,
calcium-binding protein and TRPV6, an intestinal calcium channel) and by
modulation of calcium flux across the brush border and basolateral membranes by
a means that does not require new protein synthesis. The molecular action of
1,25(OH)2D on bone has received less
attention. However, like PTH, 1,25(OH)2D
can induce RANKL in osteoblasts and proteins such as osteocalcin, which may
regulate the mineralization process. The metabolites 25(OH)D and 24,25(OH)2D
are far less potent stimulators of intestinal calcium and phosphate transport
or bone resorption. However, 25(OH)D appears to be more potent than 1,25(OH)2D
in stimulating renal reabsorption of calcium and phosphate and may be the major
metabolite regulating calcium flux and contractility in muscle. Specific
receptors for 1,25(OH)2D exist in
target tissues. However, the role and even the existence of separate receptors
for 25(OH)D and 24,25(OH)2D remain
controversial.
The receptor for 1,25(OH)2D exists in many tissues—not just bone, gut, and kidney. In these “nonclassic” tissues, 1,25(OH)2D exerts a number of actions including regulation of the secretion of PTH, insulin, and renin, dendritic cell as well as T-cell differentiation, and proliferation and differentiation of a number of cancer cells. Thus, the clinical utility of 1,25(OH)2D and its analogs is expanding.
Related Topics