Chapter: Basic & Clinical Pharmacology : Agents That Affect Bone Mineral Homeostasis
Specific Disorders Involving Bone Mineral-Regulating Hormones
SPECIFIC DISORDERS INVOLVING BONE
MINERAL-REGULATING HORMONES
PRIMARY HYPERPARATHYROIDISM
This
rather common disease, if associated with symptoms and significant
hypercalcemia, is best treated surgically. Oral phos-phate and bisphosphonates
have been tried but cannot be recom-mended. Asymptomatic patients with mild
disease often do not get worse and may be left untreated. The calcimimetic
agent cinacalcet, discussed
previously, has been approved for secondaryhyperparathyroidism and is in
clinical trials for the treatment of primary hyperparathyroidism. If such drugs
prove efficacious and cost effective, medical management of this disease will
need to be reconsidered.
HYPOPARATHYROIDISM
In
PTH deficiency (idiopathic or surgical hypoparathyroidism) or an abnormal
target tissue response to PTH (pseudohypopara-thyroidism), serum calcium falls
and serum phosphate rises. In such patients, 1,25(OH)2D
levels are usually low, presumably reflecting the lack of stimulation by PTH of
1,25(OH)2D pro-duction. The skeletons of
patients with idiopathic or surgical hypoparathyroidism are normal except for a
slow turnover rate. A number of patients with pseudohypoparathyroidism appear
to have osteitis fibrosa, suggesting that the normal or high PTH levels found
in such patients are capable of acting on bone but not on the kidney. The
distinction between pseudohypoparathy-roidism and idiopathic hypoparathyroidism
is made on the basis of normal or high PTH levels but deficient renal response
(ie, diminished excretion of cAMP or phosphate) in patients with
pseudohypoparathyroidism.
The principal
therapeutic concern is to restore normocalcemia and normophosphatemia. Vitamin
D (25,000–100,000 units three times per week) and dietary calcium supplements
have been used in the past. More rapid increments in serum calcium can be
achieved with calcitriol. Many patients treated with vitamin D experience
episodes of hypercalcemia. This complication is more rapidly reversible with
cessation of therapy using calcitriol than therapy with vitamin D. This would
be of importance to the patient in whom such hypercalcemic crises are common.
Although teriparatide (PTH 1-34) is not approved for the treatment of
hypoparathyroidism, it can be quite effective in patients who respond poorly to
calcium and vitamin D and may become the drug of choice for this condition.
NUTRITIONAL VITAMIN D DEFICIENCY OR INSUFFICIENCY
The level of vitamin D
thought to be necessary for good health is being reexamined with the
appreciation that vitamin D acts on a large number of cell types beyond those
responsible for bone and mineral metabolism. A level of 25(OH)D above 10 ng/mL
is necessary for preventing rickets or osteomalacia. However, substantial
epidemiologic and some prospective trial data indicate that a higher level,
such as 30 ng/mL, is required to optimize intestinal calcium absorption,
optimize the accrual and mainte-nance of bone mass, reduce falls and fractures,
and prevent a wide variety of diseases including diabetes mellitus,
hyperparathyroid-ism, autoimmune diseases, and cancer. However, an expert panel
for the Institute of Medicine (IOM) has recently recommended that a level of 20
ng/mL (50 nM) was sufficient for 97.5% of the population, although up to 50
ng/mL (125 nM) was considered safe. For individuals between the ages of 1–70
yrs 600 iu vitamin was thought to be sufficient to meet these goals, although
up to 4000 iu vitamin D was considered safe. These recommendations are based
primarily on data from randomized placebo controlled clinical trials (RCT) that
evaluated falls and fractures; data sup-porting the non skeletal effects of
vitamin D were considered too preliminary to be used in their recommendations
because of lack of RCT for these other actions. The lower end of these
recom-mendations has been considered too low and the upper end too restrictive
by a number of vitamin D experts, but the call for better clinical data
especially for the non skeletal actions is well taken. These guidelines—at
least with respect to the lower recommended levels of vitamin D
supplementation—are unlikely to correct vitamin D deficiency in individuals
with obesity, dark complex-ions, limited capacity for sunlight exposure, or
malabsorption. Furthermore, a large body of data from animal and cell studies
as well and epidemiologic associations support a large range of beneficial
actions of vitamin D that with adequate RCT data may alter these IOM
recommendations. Vitamin D deficiency or insufficiency can be treated by higher
dosages (4000 units per day or 50,000 units per week for several weeks). No
other vitamin D metabolite is indicated. Because the half-life of vitamin D3 metab-olites in blood
is greater than that of vitamin D2, there may be some advantage to using vitamin D3 supplements, although
when administered on a daily or weekly schedule these differences may be moot.
The diet should also contain adequate amounts of cal-cium and phosphate.
CHRONIC KIDNEY DISEASE
The major sequelae of
chronic kidney disease that impact bone mineral homeostasis are deficient
1,25(OH)2D production,
reten-tion of phosphate with an associated reduction in ionized calcium levels,
and the secondary hyperparathyroidism that results from the parathyroid gland
response to lowered serum ionized calciumand low 1,25(OH)2D. FGF23 levels are
also increased in this disorder in part due to the increased phosphate, and
this can fur-ther reduce 1,25(OH)2D production by the kidney. With impaired
1,25(OH)2D production, less calcium is absorbed from the intes-tine and less
bone is resorbed under the influence of PTH. As a result hypocalcemia usually
develops, furthering the development of secondary hyperparathyroidism. The
bones show a mixture of osteomalacia and osteitis fibrosa.
In contrast to the
hypocalcemia that is more often associated with chronic kidney disease, some
patients may become hypercal-cemic from overzealous treatment with calcium.
However, the most common cause of hypercalcemia is the development of severe
secondary (sometimes referred to as tertiary) hyperparathyroidism. In such
cases, the PTH level in blood is very high. Serum alkaline phosphatase levels
also tend to be high. Treatment often requires parathyroidectomy. A less common
circumstance leading to hyper-calcemia is development of a form of bone disease
characterized by a profound decrease in bone cell activity and loss of the
calcium buffering action of bone (adynamic bone disease). In the absence of
kidney function, any calcium absorbed from the intestine accu-mulates in the
blood. Such patients are very sensitive to the hyper-calcemic action of
1,25(OH)2D. These individuals
generally have a high serum calcium but nearly normal alkaline phosphatase and
PTH levels. The bone in such patients may have a high aluminum content,
especially in the mineralization front, which blocks nor-mal bone
mineralization. These patients do not respond favorably to parathyroidectomy.
Deferoxamine, an agent used to chelate iron , also binds aluminum and is being
used to treat this disorder. However, with the reduction in use of
aluminum-containing phosphate binders, most cases of adynamic bone dis-ease are
not associated with aluminum deposition but are attributed to overzealous
suppression of PTH secretion.
Vitamin D Preparations
The
choice of vitamin D preparation to be used in the setting of chronic kidney
disease depends on the type and extent of bone disease and hyperparathyroidism.
Individuals with vitamin D deficiency or insufficiency should first have their
25(OH)D levels restored to normal (above 30 ng/mL) with vitamin D. 1,25(OH)2D3
(calcitriol) rapidly corrects hypocalcemia and at least partially reverses
secondary hyperparathyroidism and osteitis fibrosa. Many patients with muscle
weakness and bone pain gain an improved sense of well-being.Two analogs of
calcitriol—doxercalciferol and paricalcitol—are approved for the treatment of
secondary hyperparathyroidism of chronic kidney disease. Their principal advantage
is that they are less likely than calcitriol to induce hypercalcemia for any
given reduc-tion in PTH. Their greatest impact is in patients in whom the use
of calcitriol may lead to unacceptably high serum calcium levels.
Regardless
of the drug used, careful attention to serum calcium and phosphate levels is
required. A calcium ×
phosphate product (in mg/dL units) less than 55 is desired with both calcium
and phos-phate in the normal range. Calcium adjustments in the diet and
dialysis bath and phosphate restriction (dietary and with oral inges-tion of
phosphate binders) should be used along with vitamin D metabolites. Monitoring
of serum PTH and alkaline phosphatase levels is useful in determining whether
therapy is correcting or preventing secondary hyperparathyroidism. In patients
on dialysis, a PTH value of approximately twice the upper limits of normal is
considered desirable to prevent adynamic bone disease. Although not generally
available, percutaneous bone biopsies for quantita-tive histomorphometry may
help in choosing appropriate therapy and following the effectiveness of such
therapy, especially in cases suspected of adynamic bone disease. Unlike the
rapid changes in serum values, changes in bone morphology require months to
years. Monitoring of serum vitamin D metabolite levels is useful for
determining adherence, absorption, and metabolism.
INTESTINAL OSTEODYSTROPHY
A number of
gastrointestinal and hepatic diseases cause disordered calcium and phosphate
homeostasis, which ultimately leads to bone disease. The bones in such patients
show a combination of osteoporosis and osteomalacia. Osteitis fibrosa does not
occur, in contrast to renal osteodystrophy. The important common feature in
this group of diseases appears to be malabsorption of calcium and vitamin D.
Liver disease may, in addition, reduce the produc-tion of 25(OH)D from vitamin
D, although its importance in patients other than those with terminal liver
failure remains in dispute. The malabsorption of vitamin D is probably not
limited to exogenous vitamin D as the liver secretes into bile a substantial
number of vitamin D metabolites and conjugates that are nor-mally reabsorbed in
(presumably) the distal jejunum and ileum. Interference with this process could
deplete the body of endoge-nous vitamin D metabolites in addition to limiting
absorption of dietary vitamin D.
In mild forms of
malabsorption, high doses of vitamin D (25,000–50,000 units three times per
week) should suffice to raise serum levels of 25(OH)D into the normal range.
Many patients with severe disease do not respond to vitamin D. Clinical
experi-ence with the other metabolites is limited, but both calcitriol and
calcifediol have been used successfully in doses similar to those recommended
for treatment of renal osteodystrophy. Theoretically, calcifediol should be the
drug of choice under these conditions, because no impairment of the renal
metabolism of 25(OH)D to 1,25(OH)2D and 24,25(OH)2D exists in these patients. However, calcifediol is no longer
available in the USA. Both calcitriol and 24,25(OH)2D may be of importance
in reversing the bone dis-ease. Intramuscular injections of vitamin D would be
an alterna-tive form of therapy, but there are currently no FDA-approved
intramuscular preparations available in the USA.
As
in the other diseases discussed, treatment of intestinal osteodystrophy with
vitamin D and its metabolites should be accompanied by appropriate dietary
calcium supplementation and monitoring of serum calcium and phosphate levels.
OSTEOPOROSIS
Osteoporosis is
defined as abnormal loss of bone predisposing to fractures. It is most common
in postmenopausal women but also occurs in men. The annual direct medical cost
of fractures in older women and men in the USA is estimated to be 17–20 billion
dollars per year, and is increasing as our population ages. Osteoporosis is
most commonly associated with loss of gonadal function as in menopause but may
also occur as an adverse effect of long-term administration of glucocorticoids
or other drugs, including those that inhibit sex steroid production; as a
manifesta-tion of endocrine disease such as thyrotoxicosis or
hyperparathy-roidism; as a feature of malabsorption syndrome; as a consequence
of alcohol abuse and cigarette smoking; or without obvious cause (idiopathic).
The ability of some agents to reverse the bone loss of osteoporosis is shown in
Figure 42–5. The postmenopausal form of osteoporosis may be accompanied by
lower 1,25(OH)2D levels and reduced
intestinal calcium transport. This form of osteopo-rosis is due to reduced
estrogen production and can be treated
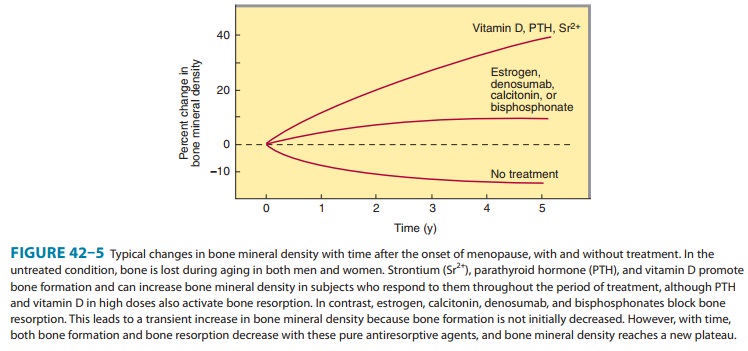
However, concern that estro-gen increases the risk of breast cancer
and fails to reduce or may actually increase the development of heart disease
has reduced enthusiasm for this form of therapy, at least in older individuals.
Bisphosphonates
are potent inhibitors of bone resorption. They increase bone density and reduce
the risk of fractures in the hip, spine, and other locations. Alendronate, risedronate, iban-dronate, and zoledronate are approved for the
treatment ofosteoporosis, using daily dosing schedules of alendronate, 10 mg/d,
risedronate, 5 mg/d, or ibandronate, 2.5 mg/d; or weekly schedules of
alendronate, 70 mg/wk, or risedronate, 35 mg/wk; or monthly schedules of
ibandronate, 150 mg/month; or quarterly (every 3 months) injections of
ibandronate, 3 mg; or annual infusions of zoledronate, 5 mg. These drugs are
effective in men as well as women and for various causes of osteoporosis.
As previously noted,
estrogen-like SERMs (selective estrogen receptor modulators) have been
developed that prevent the increased risk of breast and uterine cancer
associated with estrogen while maintaining the benefit to bone. The SERM raloxifene is approved for treatment of
osteoporosis. Like tamox-ifen, raloxifene reduces the risk of breast cancer. It
protects against spine fractures but not hip fractures—unlike bisphosphonates,
denosumab, and teriparatide, which protect against both. Raloxifene does not
prevent hot flushes and imposes the same increased risk of venous
thromboembolism as estrogen. To counter the reduced intestinal calcium
transport associated with osteoporosis, vitamin D therapy is often used in
combination with dietary calcium supple-mentation. There is no clear evidence
that pharmacologic doses of vitamin D are of much additional benefit beyond
cyclic estrogens and calcium supplementation. However, in several large
studies, vitamin D supplementation (800 IU/d) with calcium has been shown to
improve bone density, reduce falls, and prevent fractures. Calcitriol and its
analog, 1α(OH)D3, have also been shown
to increase bone mass and reduce fractures. Use of these agents for
osteoporosis is not FDA-approved, although they are used for this purpose in
other countries.
Teriparatide, the recombinant form of PTH 1-34, is approvedfor treatment of
osteoporosis. Teriparatide is given in a dosage of 20 mcg subcutaneously daily.
Teriparatide stimulates new bone formation, but unlike fluoride, this new bone
appears structurally normal and is associated with a substantial reduction in
the inci-dence of fractures. Teriparatide is approved for only 2 years of use.
Trials examining the sequential use of teriparatide followed by a
bisphosphonate after 1 or 2 years are in progress and look promis-ing. Use of
teriparatide with a bisphosphonate has not shown greater efficacy than the
bisphosphonate alone.
Calcitonin is
approved for use in the treatment of postmeno-pausal osteoporosis. It has been
shown to increase bone mass and reduce fractures, but only in the spine. It
does not appear to be as effective as bisphosphonates or teriparatide.
Denosumab,
the RANKL inhibitor, has recently beenapproved for treatment of postmenopausal
osteoporosis. It is given subcutaneously every 6 months in 60 mg doses. Like
the bisphosphonates it suppresses bone resorption and secondarily bone
formation. Denosumab reduces the risk of both vertebralcand nonvertebral
fractures with comparable effectiveness to the potent bisphosphonates.
Strontium ranelate has
not been approved in the USA for thetreatment of osteoporosis but is being used
in Europe, generally at a dose of 2 g/d.
X-LINKED & AUTOSOMAL DOMINANT HYPOPHOSPHATEMIA & RELATED DISEASES
These disorders
usually manifest in childhood as rickets and hypo-phosphatemia, although they
may first present in adults. In both X-linked and autosomal dominant
hypophosphatemia, biologi-cally active FGF23 accumulates, leading to phosphate
wasting in the urine and hypophosphatemia. In autosomal dominant
hypo-phosphatemia, mutations in the FGF23 gene replace an arginine required for
hydrolysis and result in increased FGF23 stability. X-linked hypophosphatemia
is caused by mutations in the gene encoding the PHEX protein, an endopeptidase.
Initially, it was thought that FGF23 was a direct substrate for PHEX, but this
no longer appears to be the case. Tumor-induced osteomalacia is a similar
acquired syndrome in adults that results from overexpres-sion of FGF23 in tumor
cells. The current concept for all of these diseases is that FGF23 blocks the
renal uptake of phosphate and blocks 1,25(OH)2D production leading to rickets in children
and osteomalacia in adults. Phosphate is critical to normal bone
min-eralization; when phosphate stores are deficient, a clinical and pathologic
picture resembling vitamin D–dependent rickets develops. However, affected
children fail to respond to the stan-dard doses of vitamin D used in the
treatment of nutritional rickets. A defect in 1,25(OH)2D production by the
kidney has also been noted, because the serum 1,25(OH)2D levels tend to be
low in comparison with the degree of hypophosphatemia observed. This
combination of low serum phosphate and low or low-normal serum 1,25(OH)2D provides the
rationale for treating these patients with oral phosphate (1–3 g daily) and
calcitriol (0.25–2 mcg daily). Reports of such combination therapy are
encouraging in this otherwise debilitating disease, although pro-longed
treatment often leads to secondary hyperparathyroidism.
VITAMIN D–DEPENDENT RICKETS TYPES I & II (PSEUDOVITAMIN D DEFICIENCY RICKETS & HEREDITARY VITAMIN D–RESISTANT RICKETS)
These
distinctly different autosomal recessive diseases present as childhood rickets
that do not respond to conventional doses of vitamin D. Type I vitamin
D–dependent rickets, now known as pseudovitamin D deficiency rickets, is due to
an isolated deficiency of 1,25(OH)2D
production caused by mutations in 25(OH)-D-1α-hydroxylase (CYP27B1). This
condition can be treated with vitamin D (4000 units daily) or calcitriol
(0.25–0.5 mcg daily). Type II vitamin D–dependent rickets, now known as
hereditary vitamin D resistant rickets, is caused by mutations in the gene for
the vitamin D receptor. The serum levels of 1,25(OH)2D
are very high in type II but inappropriately low for the level of calcium in
type I vitamin D–dependent rickets. Treatment with large doses of calcitriol
has been claimed to be effective in restoring normocalce-mia in some patients,
presumably those with a partially functional vitamin D receptor, although many
patients are completely resistant to all forms of vitamin D. Calcium and phosphate
infu-sions have been shown to correct the rickets in some children, similar to
studies in mice in which the VDR gene
has been deleted. These diseases are rare.
NEPHROTIC SYNDROME
Patients with
nephrotic syndrome can lose vitamin D metabolites in the urine, presumably by
loss of the vitamin D-binding protein. Such patients may have very low 25(OH)D
levels. Some of them develop bone disease. It is not yet clear what value
vitamin D therapy has in such patients, because therapeutic trials with vita-min
D (or any vitamin D metabolite) have not yet been carried out. Because the
problem is not related to vitamin D metabolism, one would not anticipate any
advantage in using the more expen-sive vitamin D metabolites in place of
vitamin D.
IDIOPATHIC HYPERCALCIURIA
Individuals with
idiopathic hypercalciuria, characterized by hyper-calciuria and nephrolithiasis
with normal serum calcium and PTH levels, have been divided into three groups:
(1) hyperabsorbers, patients with increased intestinal absorption of calcium,
resulting in high-normal serum calcium, low-normal PTH, and a secondary
increase in urine calcium; (2) renal calcium leakers, patients with a primary
decrease in renal reabsorption of filtered calcium, lead-ing to low-normal
serum calcium and high-normal serum PTH; and (3) renal phosphate leakers,
patients with a primary decrease in renal reabsorption of phosphate, leading to
increased 1,25(OH)2D production, increased intestinal calcium absorption, increased
ionized serum calcium, low-normal PTH levels, and a secondary increase in urine
calcium. There is some disagreement about this classification, and many
patients are not readily catego-rized. Many such patients present with mild
hypophosphatemia, and oral phosphate has been used with some success in
reducing stone formation. However, a clear role for phosphate in the treat-ment
of this disorder has not been established.
Therapy
with hydrochlorothiazide, up to 50 mg twice daily, or chlorthalidone, 50–100 mg
daily, is recommended. Loop diuretics such as furosemide and ethacrynic acid
should not be used because they increase urinary calcium excretion. The major
toxicity of thiazide diuretics, besides hypokalemia, hypomagnesemia, and
hyperglycemia, is hypercalcemia. This is seldom more than a biochemical observation
unless the patient has a disease such as hyperparathyroidism in which bone
turnover is accelerated. Accordingly, one should screen patients for such
disorders before starting thiazide therapy and monitor serum and urine calcium
when therapy has begun.
An
alternative to thiazides is allopurinol. Some studies indicate that
hyperuricosuria is associated with idiopathic hypercalcemia and that a small
nidus of urate crystals could lead to the calcium oxalate stone formation
characteristic of idiopathic hypercalcemia. Allopurinol, 100–300 mg daily, may
reduce stone formation by reducing uric acid excretion.
Related Topics