Chapter: Basic & Clinical Pharmacology : Agents That Affect Bone Mineral Homeostasis
Nonhormonal Agents Affecting Bone Mineral Homeostasis
NONHORMONAL AGENTS AFFECTING BONE
MINERAL HOMEOSTASIS
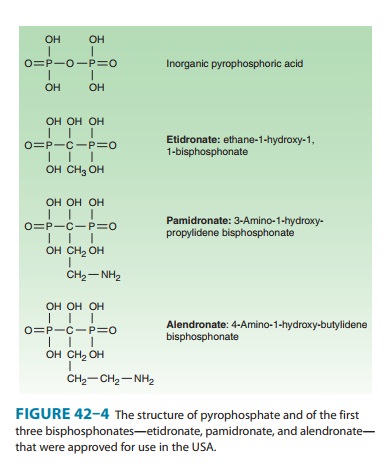
BISPHOSPHONATES
The bisphosphonates
are analogs of pyrophosphate in which the P-O-P bond has been replaced with a
nonhydrolyzable P-C-P bond (Figure 42–4). Currently available bisphosphonates
include etidronate, pamidronate, alendronate, risedronate, tiludronate, ibandronate, and zoledronate. With the development of themore potent
bisphosphonates, etidronate is seldom used.
Results from animal
and clinical studies indicate that less than 10% of an oral dose of these drugs
is absorbed. Food reduces absorption even further, necessitating their
administration on an empty stomach. A major adverse effect of oral forms of the
bis-phosphonates (risedronate, alendronate, ibandronate) is esopha-geal and
gastric irritation, which limits the use of this route by patients with upper
gastrointestinal disorders. This complication can be circumvented with
infusions of pamidronate, zoledronate, and ibandronate. Intravenous dosing also
allows a larger amount of drug to enter the body and markedly reduces the frequency
of administration (eg, zoledronate is infused once per year). Nearly half of
the absorbed drug accumulates in bone; the remainder is excreted unchanged in
the urine. Decreased renal function dic-tates a reduction in dosage. The
portion of drug retained in bone depends on the rate of bone turnover; drug in
bone often is retained for months if not years.
The bisphosphonates
exert multiple effects on bone mineral homeostasis, which make them useful for
the treatment of hyper-calcemia associated with malignancy, for Paget’s
disease, and for osteoporosis (see Box: Newer Therapies for Osteoporosis). They
owe at least part of their clinical usefulness and toxicity to their ability to
retard formation and dissolution of hydroxyapatite crystals within and outside
the skeletal system. Some of the newer bisphosphonates appear to increase bone
mineral density well beyond the 2-year period predicted for a drug whose
effects are limited to slowing bone resorption. This may be due to their other
cellular effects, which include inhibition of 1,25(OH)2D produc-tion,
inhibition of intestinal calcium transport, metabolic changes in bone cells
such as inhibition of glycolysis, inhibition of cell growth, and changes in
acid and alkaline phosphatase activity.Amino bisphosphonates such as
alendronate and risedronate inhibit farnesyl pyrophosphate synthase, an enzyme
in the mevalonate pathway that appears to be critical for osteoclast sur-vival.
The cholesterol-lowering statin drugs (eg, lovastatin), which block mevalonate
synthesis , stimulate bone for-mation, at least in animal studies. Thus, the
mevalonate pathway appears to be important in bone cell function and provides
new targets for drug development. The mevalonate pathway effects vary depending
on the bisphosphonate (ie, only amino bisphos-phonates have this property), and
may account for some of the clinical differences observed in the effects of the
various bisphos-phonates on bone mineral homeostasis.
With the exception of
the induction of a mineralization defect by higher than approved doses of
etidronate and gastric and esophageal irritation by the oral bisphosphonates,
these drugs have proved to be remarkably free of adverse effects when used at
the doses recommended for the treatment of osteoporosis. Esophageal irritation
can be minimized by taking the drug with a full glass of water and remaining
upright for 30 minutes or by using the intra-venous forms of these compounds.
Of the other complications, osteonecrosis of the jaw has received considerable
attention but is rare in patients receiving usual doses of bisphosphonates
(perhaps 1/100,000 patient-years). This complication is more frequent when high
intravenous doses of zoledronate are used to control bone metastases and
cancer-induced hypercalcemia. More recently, concern has been raised about
over-suppressing bone turnover, and case reports have appeared describing
unusual subtrochanteric (femur) fractures in patients on long-term
bisphosphonate treat-ment. This complication appears to be rare, comparable to
that of osteonecrosis of the jaw, but has led some authorities to recom-mend a
“drug holiday” after 5 years of treatment if the clinical condition warrants it
(ie, if the fracture risk of discontinuing the bisphosphonate is not deemed
high).
DENOSUMAB
Denosumab is a fully
human monoclonal antibody that binds to and prevents the action of RANKL. As
described earlier, RANKL is produced by osteoblasts. It stimulates
osteoclastogenesis via RANK, the receptor for RANKL that is present on
osteoclasts and osteoclast precursors. By interfering with RANKL function, denosumab
inhibits osteoclast formation and activity. It is at least as effective as the
potent bisphosphonates in inhibiting bone resorption and has recently been
approved for treatment of post-menopausal osteoporosis and some cancers
(prostate and breast). The latter application is to limit the development of
bone metas-tases or bone loss resulting from the use of drugs suppressing
gonadal function. Denosumab is administered subcutaneously every 6 months,
which avoids gastrointestinal side effects. The drug appears to be well
tolerated but two concerns remain. First, a number of cells in the immune
system also express RANKL, suggesting that there could be an increased risk of
infection associ-ated with the use of denosumab. Second, because the
suppression of bone turnover with denosumab is similar to that of the potent
bisphosphonates, the risk of osteonecrosis of the jaw and subtro-chanteric
fractures may be increased, although this has not been reported in the clinical
trials leading to its approval by the Food and Drug Administration (FDA).
CALCIMIMETICS
Cinacalcet is
the first representative of a new class of drugs thatactivates the
calcium-sensing receptor (CaSR). CaSR is widely distributed but has its
greatest concentration in the parathyroid gland. By activating the parathyroid
gland CaSR, cinacalcet inhib-its PTH secretion. Cinacalcet is approved for the
treatment of secondary hyperparathyroidism in chronic kidney disease and for
the treatment of parathyroid carcinoma. CaSR antagonists are also being
developed, and may be useful in conditions of hypoparathy-roidism or as a means
to stimulate intermittent PTH secretion in the treatment of osteoporosis.
PLICAMYCIN (MITHRAMYCIN)
Plicamycin is a
cytotoxic antibiotic that has been used
clinically for two disorders of bone mineral metabolism: Paget’s disease and
hypercalcemia. The cytotoxic properties of the drug appear to involve binding
to DNA and interruption of DNA-directed RNA synthesis. The reasons for its
usefulness in the treatment of Paget’s disease and hypercalcemia are unclear
but may relate to the need for protein synthesis to sustain bone resorp-tion.
The doses required to treat Paget’s disease and hypercalcemia are about one
tenth the amount required to achieve cytotoxic effects. With the development of
other less toxic drugs for these purposes, the clinical use of plicamycin is
seldom indicated.
THIAZIDE DIURETICS
The principal
application of thiazides in the treatment of bone mineral disorders is in
reducing renal calcium excretion. Thiazides may increase the effectiveness of
PTH in stimulating reabsorption of calcium by the renal tubules or may act on
calcium reabsorption secondarily by increasing sodium reabsorption in the
proximal tubule. In the distal tubule, thiazides block sodium reabsorption at
the luminal surface, increasing the calcium-sodium exchange at the basolateral
mem-brane and thus enhancing calcium reabsorption into the blood at this site
(see Figure 15–4). Thiazides have proved to be useful in reducing the
hypercalciuria and incidence of urinary stone forma-tion in subjects with
idiopathic hypercalciuria. Part of their efficacy in reducing stone formation
may lie in their ability to decrease urine oxalate excretion and increase urine
magnesium and zinc levels, both of which inhibit calcium oxalate stone
formation.
FLUORIDE
Fluoride is well
established as effective for the prophylaxis of den-tal caries and has
previously been investigated for the treatment of osteoporosis. Both
therapeutic applications originated from epide-miologic observations that
subjects living in areas with naturally fluoridated water (1–2 ppm) had less
dental caries and fewer ver-tebral compression fractures than subjects living
in nonfluoridated water areas. Fluoride accumulates in bones and teeth, where
it may stabilize the hydroxyapatite crystal. Such a mechanism may explain the
effectiveness of fluoride in increasing the resistance of teeth to dental caries,
but it does not explain its ability to promote new bone growth.
Fluoride in drinking
water appears to be most effective in preventing dental caries if consumed
before the eruption of the permanent teeth. The optimum concentration in
drinking water supplies is 0.5–1 ppm. Topical application is most effective if
done just as the teeth erupt. There is little further benefit to giving
fluo-ride after the permanent teeth are fully formed. Excess fluoride in
drinking water leads to mottling of the enamel proportionate to the
concentration above 1 ppm.
Because of the paucity
of agents that stimulate new bone growth in patients with osteoporosis,
fluoride for this disorder has been examined (see Osteoporosis, below). Results
of earlier studies indicated that fluoride alone, without adequate calcium
supple-mentation, produced osteomalacia. More recent studies, in which calcium
supplementation has been adequate, have demonstrated an improvement in calcium
balance, an increase in bone mineral, and an increase in trabecular bone
volume. Despite these promis-ing effects of fluoride on bone mass, clinical
studies have failed to demonstrate a reliable reduction in fractures, and some
studies showed an increase in fracture rate. At present, fluoride is not
approved by the FDA for treatment or prevention of osteoporosis, and it is
unlikely to be.
Adverse effects
observed—at the doses used for testing fluo-ride’s effect on bone—include
nausea and vomiting, gastrointesti-nal blood loss, arthralgias, and arthritis
in a substantial proportion of patients. Such effects are usually responsive to
reduction of the dose or giving fluoride with meals (or both).
STRONTIUM RANELATE
Strontium
ranelate is composed of two atoms of strontium bound to an organic ion, ranelic
acid. Although not yet approved for use in the USA, this drug is used in Europe
for the treatment of osteoporosis. Strontium ranelate appears to block
differentiation of osteoclasts while promoting their apoptosis, thus inhibiting
bone resorption. At the same time, strontium ranelate appears to promote bone
formation. Unlike bisphosphonates, denosumab, or teriparatide, this drug
increases bone formation markers while inhibiting bone resorp-tion markers.
Large clinical trials have demonstrated its efficacy in increasing bone mineral
density and decreasing fractures in the spine and hip. Toxicities reported thus
far are similar to placebo.
Related Topics