Chapter: Basic & Clinical Pharmacology : Aminoglycosides & Spectinomycin
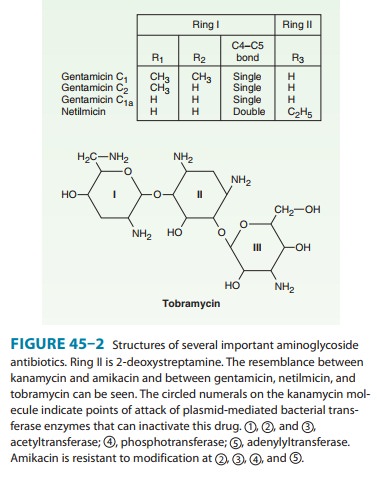
Tobramycin
TOBRAMYCIN
This aminoglycoside
(Figure 45–2) has an antibacterial spec-trum similar to that of gentamicin.
Although there is some cross-resistance between gentamicin and tobramycin, it
is unpredictable in individual strains. Separate laboratory susceptibility
tests are therefore necessary.
The pharmacokinetic
properties of tobramycin are virtually identical with those of gentamicin. The
daily dose of tobramycin is 5–6 mg/kg intramuscularly or intravenously,
traditionally divided into three equal amounts and given every 8 hours. Monitoring
blood levels in renal insufficiency is an essential guide to proper dosing.
Tobramycin has almost
the same antibacterial spectrum as gentamicin with a few exceptions. Gentamicin
is slightly more active against S
marcescens, whereas tobramycin is slightly more active against P aeruginosa; Enterococcus faecalis is susceptible to both gentamicin and
tobramycin, but E faecium is
resistant to tobramycin. Gentamicin and tobramycin are otherwise
inter-changeable clinically.
Like other
aminoglycosides, tobramycin is ototoxic and neph-rotoxic. Nephrotoxicity of
tobramycin may be slightly less than that of gentamicin, but the difference is
clinically inconsequential.
Tobramycin
is also formulated in solution (300 mg in 5 mL) for inhalation for treatment of
P aeruginosa lower respiratory tract
infections complicating cystic fibrosis. The drug is recommended as a 300-mg
dose regardless of the patient’s age or weight for administration twice daily
in repeated cycles of 28 days on ther-apy, followed by 28 days off therapy.
Serum concentrations 1 hour after inhalation average 1 mcg/mL; consequently,
nephrotoxicity and ototoxicity rarely occur. Caution should be used when
admin-istering tobramycin to patients with preexisting renal, vestibular, or
hearing disorders.
Related Topics