Chapter: Basic & Clinical Pharmacology : Aminoglycosides & Spectinomycin
Gentamicin
GENTAMICIN
Gentamicin
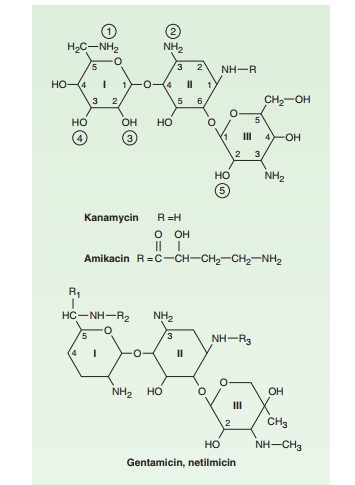
is an aminoglycoside (Figure 45–2) isolated from Micromonospora purpurea. It is effective against both gram-positiveand
gram-negative organisms, and many of its properties resemble those of other
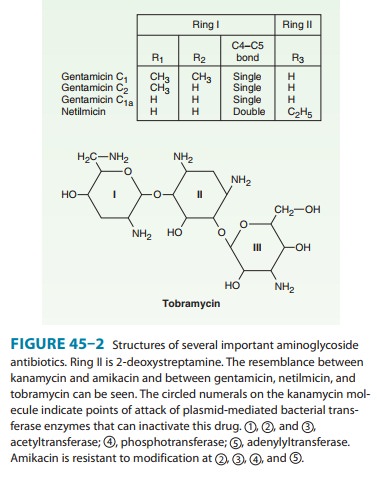
aminoglycosides. Sisomicin is very
similar to the C1a component
of gentamicin.
Antimicrobial Activity
Gentamicin
sulfate, 2–10 mcg/mL, inhibits in vitro many strains of staphylococci and
coliforms and other gram-negative bacteria. It is active alone, but also as a
synergistic companion with β-lactam antibiotics, against Escherichia coli, Proteus, Klebsiella
pneumoniae,Enterobacter, Serratia,
Stenotrophomonas, and other gram-negativerods that may be resistant to
multiple other antibiotics. Like all aminoglycosides, it has no activity
against anaerobes.
Resistance
Streptococci
and enterococci are relatively resistant to gentamicin owing to failure of the
drug to penetrate into the cell. However, gentamicin in combination with
vancomycin or a penicillin pro-duces a potent bactericidal effect, which in
part is due to enhanced uptake of drug that occurs with inhibition of cell wall
synthesis. Resistance to gentamicin rapidly emerges in staphylococci during
monotherapy owing to selection of permeability mutants. Ribosomal resistance is
rare. Among gram-negative bacteria, resistance is most commonly due to
plasmid-encoded aminoglycoside-modifying enzymes. Gram-negative bacteria that
are gentamicin-resistant usu-ally are susceptible to amikacin, which is much
more resistant to modifying enzyme activity. The enterococcal enzyme that
modifies gentamicin is a bifunctional enzyme that also inactivates amikacin,
netilmicin, and tobramycin, but not streptomycin; the latter is modified by a
different enzyme. This is why some gentamicin-resistant enterococci are
susceptible to streptomycin.
Clinical Uses
A. Intramuscular or Intravenous
Administration
Gentamicin is used
mainly in severe infections (eg, sepsis and pneumonia) caused by gram-negative
bacteria that are likely to be resistant to other drugs, especially P aeruginosa, Enterobacter sp, Serratia
marcescens, Proteus sp, Acinetobacter sp, and Klebsiella sp.It usually is used in
combination with a second agent because an aminoglycoside alone may not be
effective for infections outside the urinary tract. For example, gentamicin
should not be used as a single agent to treat staphylococcal infections because
resistance develops rapidly. Aminoglycosides also should not be used for
single-agent therapy of pneumonia because penetration of infected lung tissue
is poor and local conditions of low pH and low oxygen tension contribute to
poor activity. Gentamicin 5–6 mg/kg/d traditionally is given intravenously in
three equal doses, but once-daily administration is just as effective for some
organisms and less toxic (see above).
Gentamicin, in
combination with a cell wall-active antibiotic, is also indicated in the
treatment of endocarditis caused by gram-positive bacteria (streptococci,
staphylococci, and enterococci). The synergistic killing achieved by
combination therapy may achieve bactericidal activity necessary for cure or
allow for the shortening of the duration of therapy. The doses of gentamicin
used for synergy against gram-positive bacteria are lower than tra-ditional
doses. Typically the drug is administered at a dose of 3 mg/ kg/day in three
divided doses. Peak levels should be approximately 3 mcg/mL, while trough
levels should be <
1 mcg/mL. There are limited data to support administering the 3-mg/kg dose as a
single daily injection in the treatment of streptococcal endocarditis.
B. Topical and Ocular
Administration
Creams,
ointments, and solutions containing 0.1–0.3% gentami-cin sulfate have been used
for the treatment of infected burns, wounds, or skin lesions and in attempts to
prevent intravenous catheter infections. The effectiveness of topical
preparations for these indications is unclear. Topical gentamicin is partly
inacti-vated by purulent exudates. Ten mg can be injected subconjuncti-vally
for treatment of ocular infections.
C. Intrathecal Administration
Meningitis
caused by gram-negative bacteria has been treated by the intrathecal injection
of gentamicin sulfate, 1–10 mg/d. However, neither intrathecal nor
intraventricular gentamicin was beneficial in neonates with meningitis, and
intraventricular gen-tamicin was toxic, raising questions about the usefulness
of this form of therapy. Moreover, the availability of third-generation
cephalosporins for gram-negative meningitis has rendered this therapy obsolete
in most cases.
Adverse Reactions
Nephrotoxicity is
usually reversible and mild. It occurs in 5–25% of patients receiving
gentamicin for longer than 3–5 days. Such toxicity requires, at the very least,
adjustment of the dosing regi-men and should prompt reconsideration of the need
for the drug,particularly if there is a less toxic alternative agent.
Measurement of gentamicin serum levels is essential. Ototoxicity, which tends
to be irreversible, manifests itself mainly as vestibular dysfunction. Loss of
hearing can also occur. The incidence of ototoxicity is in part genetically
determined, having been linked to point muta-tions in mitochondrial DNA, and
occurs in 1–5% for patients receiving gentamicin for more than 5 days.
Hypersensitivity reac-tions to gentamicin are uncommon.
Related Topics