Chapter: Plant Biochemistry: Biotechnology alters plants to meet requirements of agriculture, nutrition and industry
Ti-Plasmids are used as transformation vectors
Ti-Plasmids are used as transformation vectors
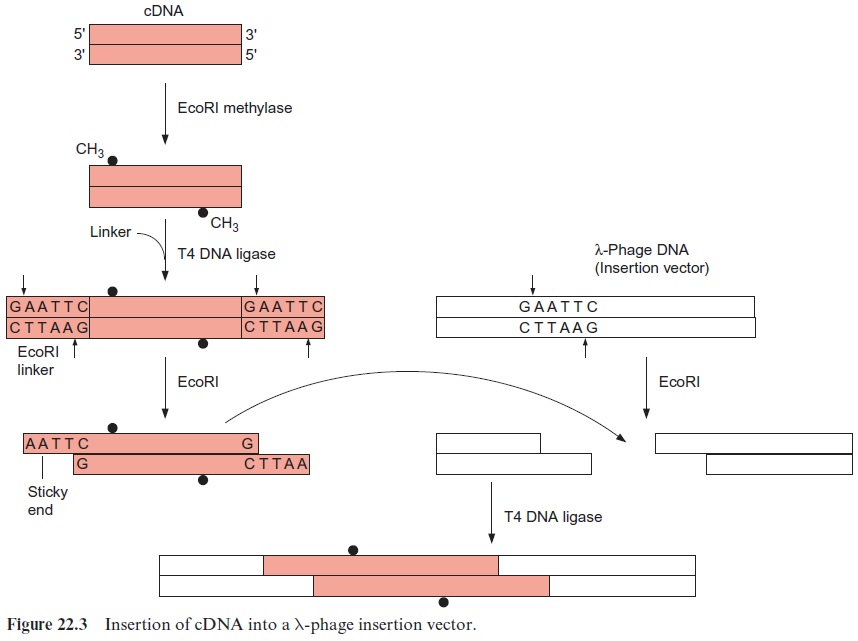
Its ability to transform plants has made A. tumefaciens an excellent tool for integrating foreign genes in their functional state in a plant genome. It was necessary, however, to modify Ti-plasmids before they could be used as vectors (Fig. 22.14). The genes for auxin and cytokinin synthesis were removed to prevent tumor growth in the transformed plants. Since synthe-sis of an opine is unnecessary for a transgenic plant and would be a burden on its metabolism, the genes for opine synthesis were also removed. Thus the T-DNA is defined only by the two border sequences. In order to insert a foreign gene between these two border sequences, it was necessary to incorporate a DNA sequence containing cleavage sites for several restric-tion endonucleases (known as a polylinker sequence or a multicloning site) within the T-DNA region of the Ti-plasmid. The Ti-plasmid could then be cleaved in the T-DNA region by a certain restriction endonuclease. A for-eign DNA sequence, excised by the same restriction endonuclease, can be inserted in this cleavage site (see Fig. 22.3). Since the polylinker sequence contains cleavage sites for several restriction endonucleases, several DNA molecules can be inserted sequentially into the T-DNA. In Figure 22.14, a promoter, enabling the expression of the inserted DNA in the host cell, is located to the left of the cleavage site.
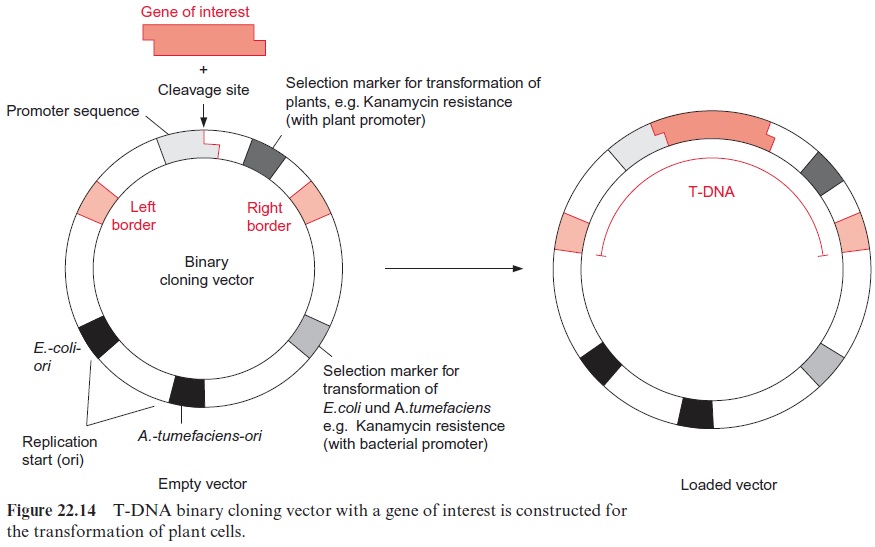
In modern transformation systems, vectors derived from the Ti-plasmid no longer contain any vir genes and are therefore unable to transform a plant cell on their own. For transformation they require the assistance of a second so-called helper plasmid, which contains the vir genes, but no T-DNA and is therefore unable to transform a plant on its own. Those A. tumefaciens strains used nowadays in biotechnological applications pos-sess the helper plasmid (Fig. 22.15). The vector used for the transforma-tion is referred to as binary cloning vector. A large variety of such has been designed for special applications and is available commercially.
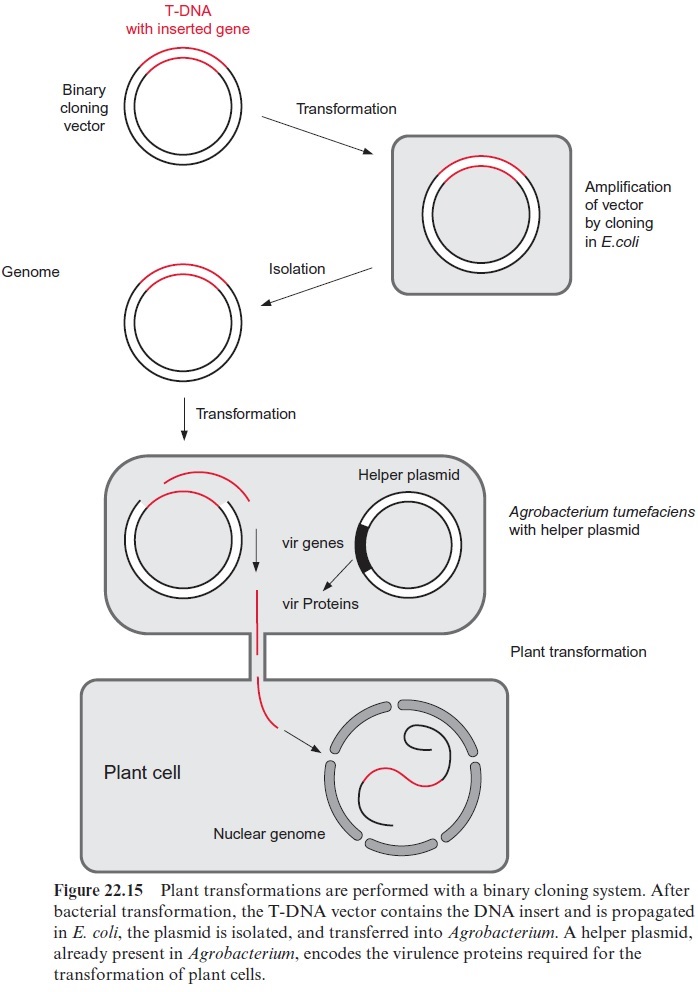
In order to transform a plant, a sufficient amount of vector-DNA, con-taining the gene to be transferred, has to be isolated. It proved to be advan-tageous to first amplify the vectors by cloning them in E. coli (Fig. 22.15). Since E. coli does not recognize the replication start site of the natural Ti-plasmid (A. tumefaciens-ori), a second replication start (E. coli-ori) is intro-duced into the plasmid.
The plasmid is provided with a selection marker in order to select those E. coli cells that have been transformed by the Ti-plasmid. For this a gene encoding neomycin phosphotransferase is frequently used. This enzyme degrades the antibiotickanamycin, thus rendering the cell resistant to this antibiotic. The kanamycin resistance gene is linked to a bacterial promoter and therefore its expression is limited to E. coli cells. When kanamycin is added to the culture medium of the bacteria, only the transformed bacteria survive, since they are protected against the antibiotic due to the resistance gene on the vector. Thus, the Ti-plasmid can be propagated efficiently by cloning in E. coli.
A new plant is regenerated after the transformation of a leaf cell
After transformation, the few transformed plant cells are selected from the large number of nontransformed cells by another selection marker pro-vided by the T-DNA vector. In most cases the kanamycin resistance gene described previously is also used for this purpose, but in this specific case provided with a plant promoter. Since kanamycin is very seldom used in medicine the use of a kanamycin resistance gene in generating transgenic plants appears to be advantageous.
A. tumefaciens attacks plants at wounded areas. Leaf discs therefore, with their cut edges, are good targets for a trans-formation with A. tumefaciens (Fig. 22.16). The leaf discs are immersed in a suspension of A. tumefaciens cells that comprise the binary and helper vectors. After a short time, the discs are transferred to a culture medium containing agarose, which, besides nutrients, contains the phytohormones cytokinin and auxin which induce cells of the leaf disc to grow to a callus. The addition of the antibiotics, e.g., kanamycin, only allows the growth of transformed cells. The cut edges of the leaf discs are the site where the calli of the transformed cells develop. When the concentrations of cytokinin and auxin are appropriate, these calli can be propagated in tissue culture. In this way transformed plant cells can be kept and propagated in tissue cul-ture for very long periods of time. If required, new plants can be regener-ated from these tissue cultures.
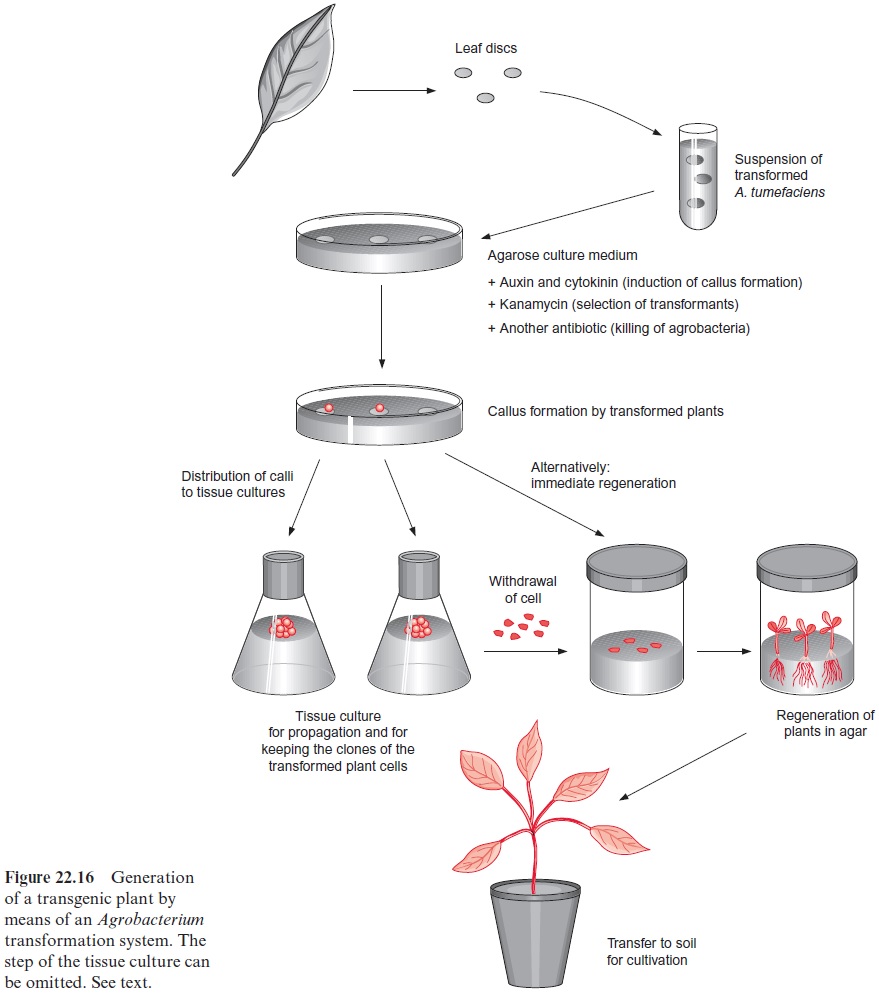
To regenerate new plants, cells of the callus culture are transferred to a culture medium containing more cytokinin than auxin, and this hormonal imbalance induces the callus to develop shoots. Root growth is then stimu-lated by transferring the shoots to a culture medium containing more auxin than cytokinin. After plantlets with roots were grown to an appropriate size, they can be transplanted to soil, where in most cases they develop into normal plants, capable of reproduction by flowering and seed production.
The pioneer work of Jeff Schell, Marc van Montagu, Patricia Zambryski, Robert Horsch, and several others has developed the A. tumefaciens transformation system to a very easy method for transferring foreign genes to cells of higher plants. Nowadays it is often possible for even students to produce several hundred different transgenic tobacco plants with no great difficulty.
Using this method, well over 100 different plant species have been trans-formed successfully. Initially, it was very difficult or even impossible to transform monocot plants with the Agrobacterium system. Recently, this transformation method has been improved to such an extent, that it can now also be successfully applied to transform several monocots, such as rice. A successful adaption of the method is the agroinjection in which the agrobacteria suspension is injected into the stigma of flowers. This method has been used for altering the gene expression in tomato fruits. An alterna-tive way to transform plant cells is a physical gene transfer, the most suc-cessful being the bombardment of plant cells by microprojectiles.
Plants can be transformed by a modified shotgun
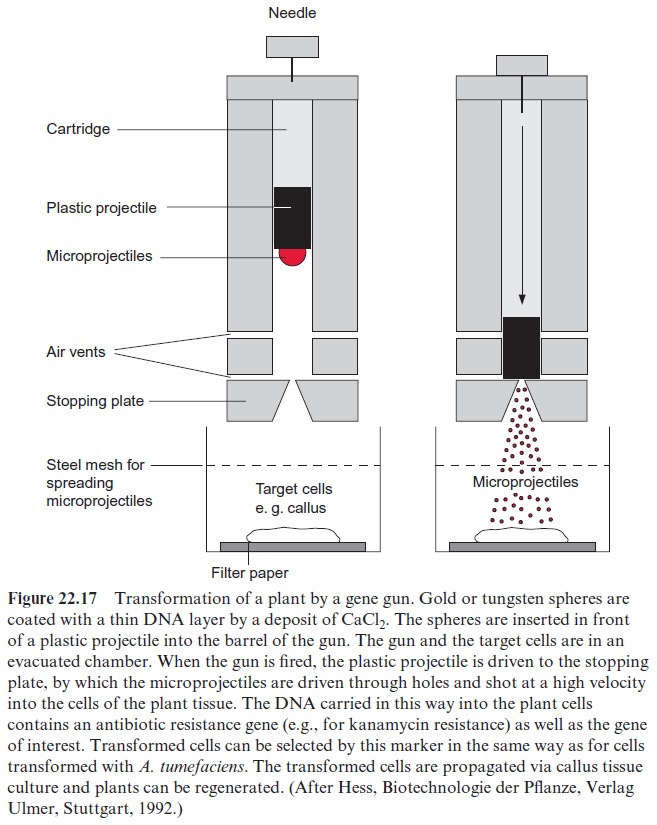
Transformation by bombardment of plant cells with microprojectiles was developed in 1985. The microprojectiles are small spheres of tungsten or gold with a diameter of 1 to 4 µm, which are coated with DNA. A gene gun (similar to a shotgun) is used to shoot the pellets into plant cells (Fig. 22.17). Initially, gunpowder was used as propellant, but nowadays themicroprojectiles are often accelerated by compressed air, helium, or other gases. The target materials include calli, embryonic tissues, and leaves. In order to penetrate the cell wall of the epidermis and mesophyll cells, the velocity of the projectiles must be very high and can reach about 1,500 km/h in a vacuum chamber. The cells in the center of the line of fire may be destroyed and killed during such a strong bombardment, but, because the projectiles are so small, the cells nearer the periphery survive. The DNA transferred to the cells by these projectiles can be integrated not only into the nuclear genome, but also into the genome of mitochondria and chloro-plasts. Therefore, this method allows the transformation of mitochondria and chloroplasts. In some plants, the gene gun works especially well. Thus, by bombardment of embryonic callus cells of sugarcane, routinely up to 10 to 20 different transformed plant lines can be obtained with one shot.
Protoplasts can be transformed by the uptake of DNA
The transformation of protoplasts is another way to transfer foreign genetic information into a plant cell. Protoplasts can be obtained from plant tissues by digestion of the cell walls . Protoplasts are able to take up foreign DNA in the presence of CaCl2 and polyethylene glycol, and often integrate the DNA into their genomes. This transformation resembles that of bacte-ria by plasmids. During protoplast transformation, the gene to be transferred is linked with a selection marker encoding resistance to an antibiotic. After the antibiotic has been added, only the transformed protoplasts survive. In prin-ciple, the protoplasts of all plants can be transformed in this way, since there is no host specificity involved, as in the case of transformation by A. tumefa-ciens. However, the use of this method is applied successfully to only some plant species, e.g., rice and maize, where it has been possible to regenerate intact fertile plants from protoplasts. In fact it is the regeneration of plant which limits the application of this method.
Plastid transformation to generate transgenic plants is advantageous for the environment
Recently, the transformation of chloroplasts has gained importance. Transgenic plants obtained by transformation of the nuclear genome (e.g., by the Ti-plasmid) could pass their genetic information via pollen to cul-tivars in neighboring fields or in some cases also to related wild plants. This can lead to undesirable cross-breeding (e.g., causing the generation of herbicide-resistant weeds in the neighborhood of herbicide-resistant culti-vars). This problem can be avoided when the genetic transformations are performed in the plastid genome of the plant. Since in most cases the plas-tid genome ismaternally inherited, the genetic alteration will not spread to other plants via pollen transfer (pollination).
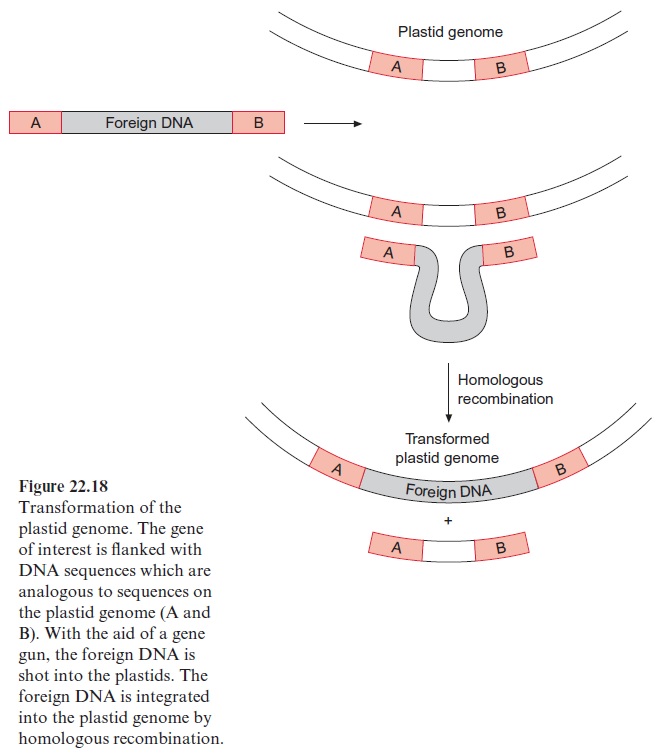
The gene gun is usually used for the transformation of chloroplasts. The foreign DNA that is to be integrated into the plastid genome is provided at both ends with sequences, which are identical to sequences in the plas-tid genome (Fig. 22.18). After the foreign DNA has entered the plastids, it can be integrated into the plastid genome by homologous recombination at a site defined by the sequences at both ends. Whereas in the plant nuclear genome homologous recombinations are rare events, these occur frequently in the plastid genome. In this way random mutations are avoided, which occur when Ti-plasmids are used for transformation of the nuclear genome. A drawback of plastid transformation is, however, that plant cells contain many plastids, each with 10 to 100 genomes. By repetitive selection and regeneration, it is possible to achieve transgenic lines in which practically all the plastid genomes have integrated the foreign DNA (transplastome plants). Plants in which each cell contains many hundred copies of a for-eign gene can be cultivated. This has the advantage that these transformed plants can produce large amounts of foreign proteins (up to 46% of the sol-uble protein), which might be relevant when the plants are to be used for the synthesis of defense compounds or pharmaceuticals.
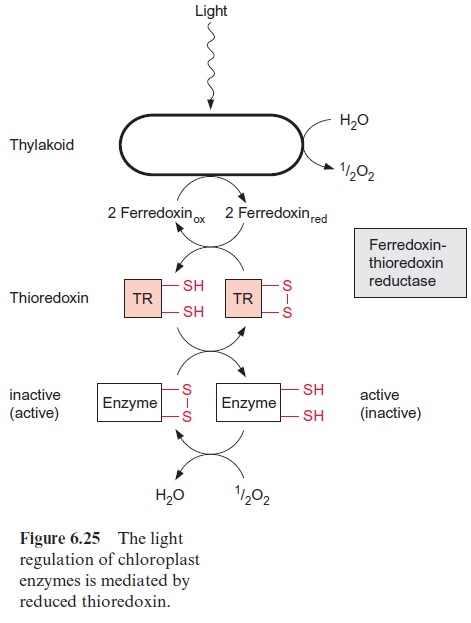
Another advantage of the plastid transformation is that plastid genes can be transcribed polycistronically. While the DNA present in the nucleus usually is transcribed to monocistronic mRNAs, which are then translated into only one protein, most of the plastid genes are transcribed to polycis-tronic mRNAs, which encode several proteins and afterwards are processed to single translatable mRNAs. This property of the plastid transcription allows the integration of several foreign genes (e.g., for a synthesis pathway) in one step into the plastid genome. It should be noted, however, that in certain cases this can also be achieved by the transformation of the nuclear genome. Another advantage of the plastid transformation is that proteins with a disulfide bridge can be formed in the plastids, which is not possible in the cytosol. Chloroplast enzymes are regulated by the oxidation of adjacent -SH groups (Fig. 6.25). Because of this ability the plastid compartment is well suited to produce, after genetic transformation, animal proteins, such as antibodies or oral vaccines, where disulfide bridges are responsible for the correct folding. The expression of genes originating from bacteria, e.g., Bt toxin , is made easy since bacteria and chloroplasts employ the same triplet codons. Transplastome tobacco plants have been generated. The plastid transformation of other crop plants is still a big challenge, as it would allow generating transformants which har-monize with the environment, as they cannot spread their foreign genes to other plants by pollen.
Related Topics