Chapter: Mechanical : Advanced IC Engines : Spark Ignition Engines
Thermodynamic analysis of SI engine combustion process
Thermodynamic analysis of SI engine combustion
process
Burned and Unburned Mixture States
The gas
pressure, temperature, and density change as a result of changes in volume due
to piston motion. During combustion, the cylinder pressure increases due to the
release of the fuel's chemical energy. As each element of fuel-air mixture
bums, its density decreases by about a factor of four. This combustion-produced
gas expansion compresses the unburned mixture ahead of the flame and displaces
it toward the combustion chamber walls. The combustion-produced gas expansion
also compresses those parts of the charge which have already burned, and
displaces them back toward the spark plug. During the combustion process, the
unburned gas elements move away from the spark plug; following combustion,
individual gas elements move back toward the spark plug. Further, elements of
the unburned mixture which burn at different times have different pressures and
temperatures just prior to combustion, and therefore end up at different states
after combustion. The thermodynamic state and composition of the burned gas is,
therefore, non-uniform. A first law analysis of the spark-ignition engine
combustion process enables us to quantify these gas states. Work transfer
occurs between the cylinder gases and the piston (to the gas before TC; to the
piston after TC). Heat transfer occurs to the chamber walls, primarily from the
burned gases. At the temperatures and pressures typical of spark-ignition
engines it is a reasonable approximation to assume that the volume of the
reaction zone where combustion is actually occurring is a negligible fraction
of the chamber volume even though the thickness of-the turbulent flame may not
be negligible compared with the chamber dimensions (see Sec. 9.3.2). With
normal engine operation, at any point in time or crank angle, the pressure
throughout the cylinder is close to uniform. The conditions in the burned and
unburned gas are then determined by conservation of mass:
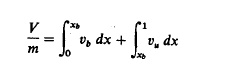
and
conservation of energy:
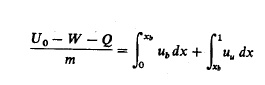
where V
is the cylinder volume, m is the mass of the cylinder contents, o is the
specific volume, xb is the mass fraction burned, Uo is the internal energy
of the cylinder contents at some reference point 80, u is the specific internal energy, W is the work done on the
piston, and Q is the heat transfer to the walls. The subscripts u
and b denote unburned and burned gas properties, respectively. The work and
heat transfers are
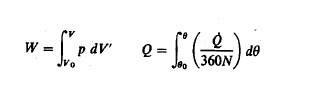
where 0
is the instantaneous heat-transfer rate to the chamber walls. To proceed
further, models for the thermodynamic properties of the burned and unburned
gases are required. Several categories of models are described in Chap. 4.
Accurate calculations of the state of the cylinder gases require an equilibrium
model (or good approximation to it) for the burned gas and an ideal gas mixture
model (of frozen composition) for the unburned gas. However, useful
illustrative results can be obtained by assuming that the burned and unburned
gases are different ideal gases, each with constant specific heat.
Related Topics