Chapter: Pharmaceutical Drug Analysis: Iodimetric and Iodometric Titrations
Theory - Iodimetric and Iodometric Titrations
THEORY
In iodimetry,
quantitative oxidation of reducing agents, such as arsenious acid (H2AsO3)
may be carried out by employing standard solutions of iodine as shown under :

This type of assay is known as ‘direct method of iodimetry’.
In another situation, a known excess quantity of standard
iodine solution is added in the substance (a reducing agent) to be assayed and
then the excess iodine may be titrated with the help of standard sodium
thiosulphate solution, such as : the estimation of sodium bisulphite :
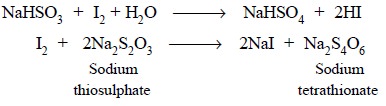
This category of assay is termed as ‘residual method of iodimetry’.
In iodometry,
an equivalent amount of iodine is liberated when the given sample of an
oxidizing agent oxidizes potassium iodide in an acidic medium, for example :
the determination of cupric sulphate (CuSO4) :
2CuSO4 + 4 KI → 2CuI ↓ + I2
+ 2K2SO4
Consequently, the equivalent amount of iodine generated
by the above reaction may be conveniently assayed by titration against a
standard sodium thiosulphate solution. In this context a point of caution must
be observed while KI is being oxidized under a strongly acidic medium so as to
avoid simultaneous oxidation of the iodide by atmospheric oxygen that may
result high erroneous titer values leading to false estimations.
It is, however, pertinent to mention here that iodometric
assays are never performed in a strongly basic medium, because of the fact that
the reaction between I2 and OH– produces hypoiodide and
iodate ions respectively as shown below :
I2 + OH–
→ HI + IO–
3IO– → IO3– + 2I
The said two ions partially oxidize thiosulphate to a
higher oxidation form, such as sulphate (SO42–) thereby
the stoichiometry achieved is always false.
Related Topics