Chapter: Pharmaceutical Drug Analysis: Iodimetric and Iodometric Titrations
Argentometric Precipitation Methods: Theory
THEORY
In the precipitation reaction involving chloride and
silver nitrate, the addition of even a small quantity of the latter shall
effect precipitation of AgCl provided that Ksp
has been exceeded significantly. At this juncture, the concentrations of both
Ag+ and Cl– are related by the solubility-product
equilibrium constant thus, we have :
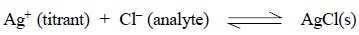
Chromate ion concentration required to initiate the
precipitation of Ag2CrO4 commences at the equivalence
point and may be calculated with the solubility products for AgCl and Ag2CrO4
:
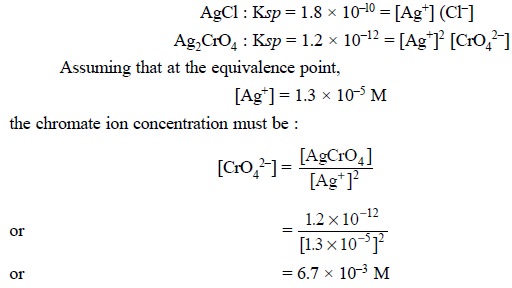
In actual practice, the concentration of chromate
produces an intense yellow colour to such an extent that the end point is
masked. Therefore, normally concentrations of 5 × 10 –3 M are
employed in analytical procedures. It suggests that [Ag+] shall be
> 1.3 × 10 –5 M at the end-point thereby introducing a positive
determinate error. However, it has been proved experimentally that even with
concentrations as low as 2 × 10 –3 M, the extent of error caused is
negligibly small.
Adsorption-coprecipitation phenomenon using fluorescein,
dichlorofluorescein and tetrabromofluo-rescein (eosin) essentially impart the
fluoresceinate ion that is absorbed on the AgCl particles. At the equiva-lence
point, the AgCl particles change from white to pink due to the coprecipitation
of silver fluoresceinate. In short, the adsorption indicator method is quite rapid
and capable of providing very accurate results for the estimation of Cl–
with AgNO3.
Furthermore, Br–, I– and SCN–
ions can also be titrated with AgNO3 employing eosin as an
adsorption indicator.
Related Topics