Chapter: Pharmaceutical Drug Analysis: Iodimetric and Iodometric Titrations
Iodometric Assays
IODOMETRIC ASSAYS
In iodometric determinations the pharmaceutical substance
oxidizes KI in an acidic medium to produce an equivalent quantity of iodine
that may be assayed by titration with a standard solution of sodium
thiosulphate.
1. Chlorinated Lime
Chlorinated lime or bleaching powder, CaOCl2,
contains about 30% w/w of available chlorine.
Theory : Chlorinated lime reacts with
acetic acid to produce a mole each of calcium acetate, hydro-chloric acid and
hydrochlorous acid. The two acids interact to give water and chlorine, and the
latter reacts with HI to liberate iodine that can be estimated by titrating
with 0.1 N sodium thiosulphate solution. The various reactions involved may be
expressed as given below :

Materials Required : Chlorinated lime : 4 g ;
dilute acetic acid : 5 ml ; potassium iodide : 3 g ; acetic acid : 5 ml ; 0.1 N
sodium thiosulphate solution.
Procedure : Weigh accurately 4.0 g of
chlorinated lime and triturate it in a glass-pestle-mortar with a little DW. Transfer the paste
quantitatively into a 1 litre volumetric flask and shake thoroughly. Take a 100
ml volumetric flask, rinse it with a small quantity of the suspension from the
1 litre flask and finally fill it up with the suspension. Rinse out a 250 ml
iodine flask containing a little dilute acetic acid and a little of the
suspension from the 1-litre flask in order to oxidise any inorganic substance
present in the iodine flask. Finally, wash it thoroughly with DW. Now, transfer
100 ml of the suspension completely from the 100 ml volumetric flask to the
iodine flask by washing the former repeatedly with DW. Add to it acetic acid 5
ml followed by KI 3.0 g and shake the contents of the flask thoroughly. Titrate
the liberated iodine with 0.1 N sodium thiosulphate which is equivalent to
0.003546 g of chlorine.
From this value the percentage of chlorine present in the
given sample of chlorinated lime can be calculated.
2. Ferric Ammonium Citrate
Theory : In ferric ammonium citrate it
is taken for granted that the entire iron is oxidized to the Fe2+ state and practically little Fe2+
is present. Thus, the ferric ion present in a known amount of the sample
liberates an equivalent amount of iodine from an acidified KI solution. Thus,
we have :
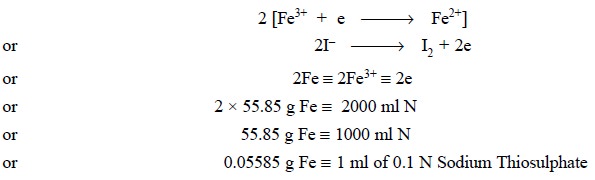
Materials Required : Ferric ammonium citrate : 0.5
g ; sulphuric acid conc. : 1 ml ; 0.1 N KMnO4 solution : 50 ml ;
hydrochloric acid : 15 ml ; potassium iodide : 2.0 g ; 0.1 N sodium
thiosulphate.
Procedure : Weigh accurately about 0.5 g
of ferric ammonium citrate and dissolve the sample in 15 ml DW. Add to it slowly 1 ml of sulphuric acid and warm gently to
attain a yellow colouration so as to decompose the iron and ammonium citrate
complex completely. Cool and add 0.1 N potassium permanganate solution dropwise
from a burette to obtain a pink colour that persists for 5 seconds. To the
resulting solution add hydrochloric acid 15 ml and potassium iodide 2.0 g,
shake well and set aside for 3 minutes so that iodine may be liberated
completely. Now, add 60 ml of water and titrate with 0.1 N sodium thiosulphate
solution while shaking the contents continuously till a colourless end-point is
achieved.
Precautions :
(i) Addition of
excess of KMnO4 solution must be avoided, since pink colour
developed shall disap-pear within a short span, which may ultimately give false
high results,
(ii) Washing
down during the course of titration must be checked rigidly in order to
maintain the right proportion of various substances in the solution,
(iii) End-point
is almost colourless, hence starch indicator can be skipped totally, and
(iv) KMnO4
oxidizes the traces of Fe2+ to Fe3+ in the sample, if
any.
3. Thyroid
Thyroxine and diidotyrosine are the two
iodine-substituted organic compounds which essentially con-stitute the active
principles present in dried thyroid gland. The latter on being subjected to
pyrolysis with anhydrous K2CO3, gives rise to an
equivalent amount of KI present in the sample. Soon after the completion of
carbonization, the crucible is cooled and the residue is extracted with water
to dissolve KI, carbonates and other soluble compounds. The resulting solution
is filtered and treated with Br2 in the presence of phosphoric acid
(H3PO4) so that complete oxidation of iodide to iodate is
caused. The following reaction takes place :

The excess of bromine is removed by warming the acidic
solution gently till the vapours show a negative test with starch-iodide paper.
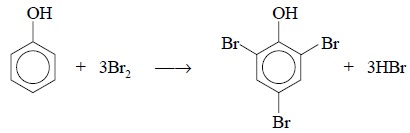
However, the residual traces of Br2 are reduced by treatment of the
resulting solution with phenol to yield the corresponding 2,4,6-tribromophenol
as shown below :
Lastly, iodate (IO3–) in a weak
acidic medium quantitatively oxidizes KI to an equivalent amount of iodine, as
expressed below :
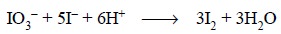
It is evident from the above equation that each
gram-atomic weight of iodine in thyroid is converted to 1 mol of iodate and
finally to 3 mol or 6 equivalent of iodine. Therefore, the equivalent weight of
the iodine present in the dried thyroid gland is 21.15 g (i.e., 1/6 × 127 At. wt. of I 2). Hence, each millilitre
of 0.01 N sodium thiosulphate is equivalent to 0.0002115 g of iodine (i.e., 0.01 × 0.02115 g).
Materials Required : Thyroid gland dried 1.0 g ;
anhydrous potassium carbonate : 17.0 g ; bromine solution (9.6 ml of Br2 and 30 g of KBr in 100 ml DW) :
7.0 ml ; dilute phosphoric acid (10% w/v) : 42.0 ml ; starch iodide paper ;
phenol solution (saturated solution of phenol in water) : 5.0 ml ; potassium
iodide solution (10% w/v in water) ; 0.01 N sodium thiosulphate solution ;
starch solution.
Procedure : Weigh accurately about 1.0 g
of dried thyroid gland in a porcelain crucible, add 7.0 g of anhydrous K2CO3,
mix thoroughly and overlay with further 10 g more of anhydrous K2CO3,
finally compact the mixture by tapping gently. Incenerate for 25 minutes at
675°—700°C in a preheated muffle furnace. Cool the contents, add 20 ml of DW,
boil gently and decant through a filter paper into a flask. Repeat the
extraction by boiling with 20 ml DW, wash the crucible and the residue on the
filter with hot water until the filtrate is about 200 ml. To it add 7.0 ml of
freshly prepared bromine solution followed by 40 ml of dilute phosphoric acid
and continue boiling slowly till starch iodide paper is no longer coloured blue
by the vapours. While boiling is in progress top up the volume to 200 ml by
adding DW at intervals. Cool and add 5 ml of phenol solution and allow to stand
for 5 minutes. Add 2 ml of dilute phosphoric acid and 5 ml of potassium iodide
solution and titrate immediately with 0.01 N sodium thiosulphate solution
employing starch solution as indi-cator towards the end-point. A blank
estimation is also carried out simultaneously and necessary correction
incorporated. Each ml 0.1 N sodium thiosulphate is equivalent to 0.0002115 g of
I.
Precautions :
(i) Potassium
carbonate should be perfectly anhydrous otherwise decrepitation would take
place caus-ing loss of material during pyrolysis,
(ii) Both the
temperature of the muffle furnace and the extent of heating should be monitored
closely, because KI is significantly volatile at an elevated temperature and
part of it may be lost due to extended heating, and
(iii) The
solution from which excess Br2 is removed by heating must be acidic,
otherwise a portion of Br2 shall be fixed in the form of potassium
hypobromite (KBrO).
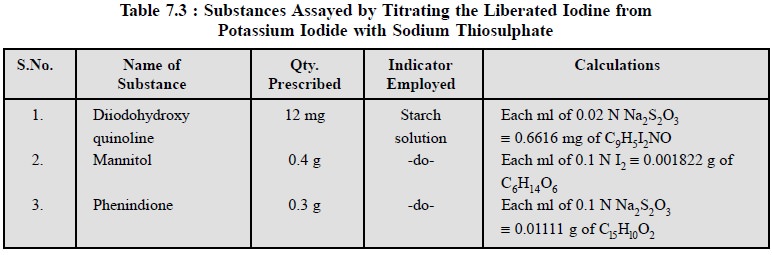
4. Cognate Assays
A few pharmaceutical substances can be assayed by
titrating the liberated iodine from potassium iodide with sodium thiosulphate
as stated in Table 7.3.
Related Topics