Chapter: Plant Biochemistry: Polysaccharides are storage and transport forms of carbohydrates produced by photosynthesis
The utilization of the photosynthesis product triose phosphate is strictly regulated
The utilization of the photosynthesis product triose phosphate is strictly regulated
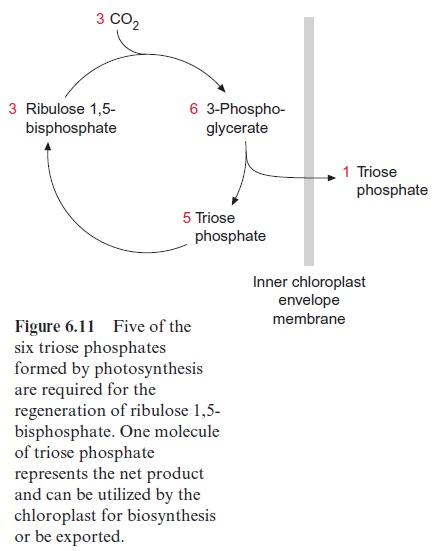
As shown in Figure 6.11, five of the six triose phosphate molecules gener-ated in the Calvin cycle are required for the regeneration of the CO2 accep-tor ribulose bisphosphate. Therefore, a maximum of one-sixth of the triose phosphate produced is available for export from the chloroplasts. In fact, due to photorespiration , the portion of available triose phos-phate is only about one-eighth of the triose phosphate synthesized in the chloroplasts. If more triose phosphate would be withdrawn from the Calvin cycle, the CO2 acceptor ribulose bisphosphate could no longer be regener-ated and the Calvin cycle would collapse. To keep the Calvin cycle running it is crucial that the withdrawal of triose phosphate does not exceed this limit. On the other hand, photosynthesis of chloroplasts can only proceed if its product triose phosphate is utilized (e.g., for synthesis of sucrose), and consequently phosphate is released. A phosphate deficiency would result in a decrease or even a total cessation of photosynthesis. Thus, it is important for a plant to match the increase of the photosynthesis rate (e.g., during strong sunlight) with the increase in the synthesis and utilization of assimi-lation products. Therefore the utilization of the triose phosphate generated by photosynthesis should be regulated in such a way that as much as possi-ble is utilized without exceeding the limit, to ensure the regeneration of the CO2 acceptor ribulose bisphosphate.
Fructose 1,6-bisphosphatase is an entrance valve of the sucrose synthesis pathway
In mesophyll cells, sucrose synthesis is normally the main consumer of triose phosphate generated by CO2 fixation. The withdrawal of triose phosphate from chloroplasts for the synthesis of sucrose is not regulated via translo-cation between chloroplast and cytosol. Due to the hydrolysis of fructose 1,6-bisphosphate and sucrose 6-phosphate, the complete reaction of sucrose synthesis (Fig. 9.14) is an irreversible process, which has a high synthetic capacity due to high enzymatic activities. Sucrose synthesis must be strictly regulated to ensure that not more than the permitted amount of triose phos-phate (see preceding paragraph) is withdrawn from the Calvin cycle.
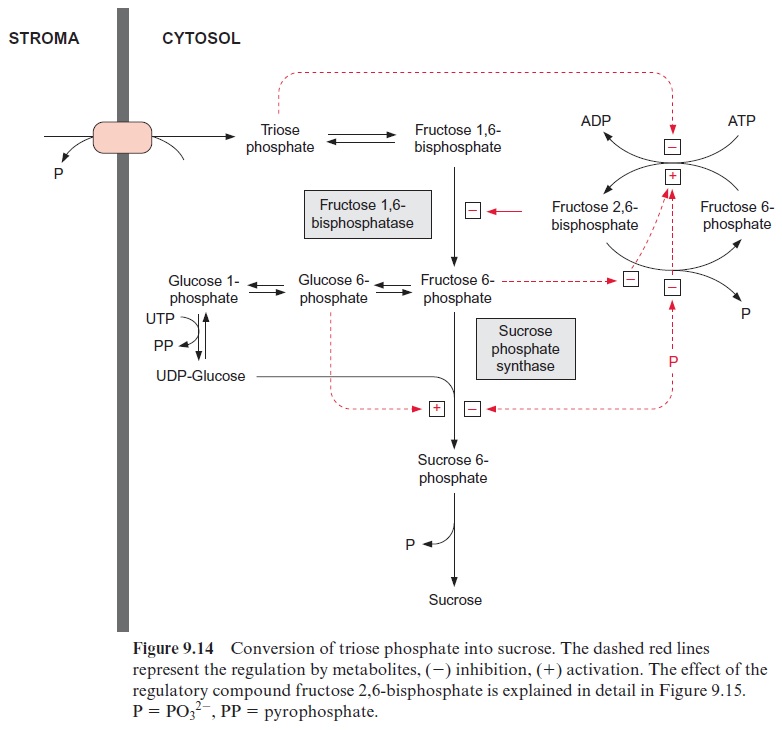
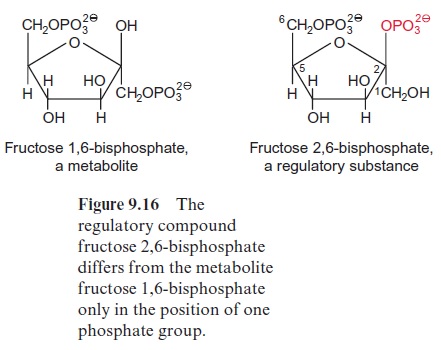
The first irreversible step of sucrose synthesis is catalyzed by the cytosolic fructose 1,6-bisphosphatase. This reaction is an important control point and is the entrance valve where triose phosphate is recruited for the synthesis of sucrose. Figure 9.15 shows how this valve is regulated. An important role is played by fructose 2,6-bisphosphate (Fru2,6BP), a regula-tory compound that differs from the metabolic fructose 1,6-bisphosphate only in the positioning of one phosphate group (Fig. 9.16).
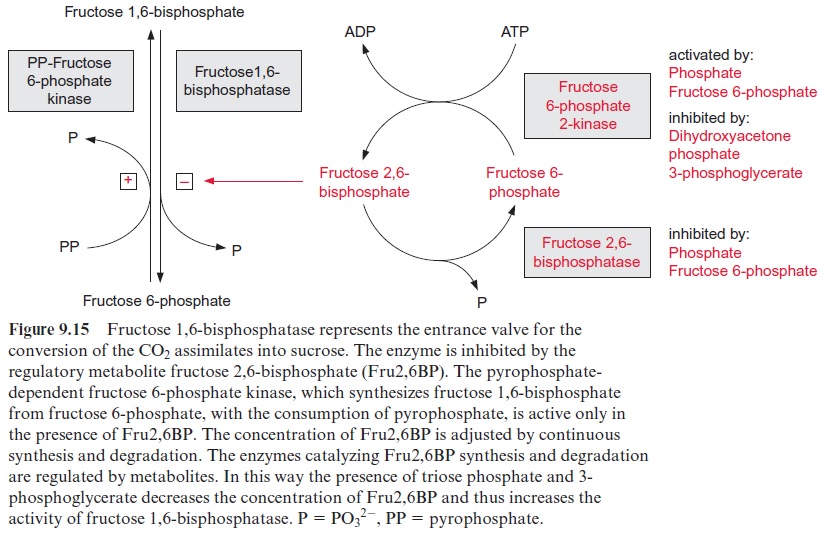
Fru2,6BP was discovered to be a potent activator of ATP-dependent fructose 6-phosphate kinase and an inhibitor of fructose 1,6-bisphosphatase in liver. Later it became apparent that Fru2,6BP has a general function in controlling glycolysis and gluconeogenesis in animals, plants, and fungi. It is a powerful regulator of cytosolic fructose 1,6-bisphosphatase in meso-phyll cells. At micromolar concentrations Fru2,6BP decreases the affinity of the enzyme towards its substrate fructose 1,6-bisphosphate. On the other hand, Fru2,6BP activates a pyrophosphate-dependent fructose 6-phosphate kinase present in the cytosol of plant cells. This enzyme is inactive when Fru2,6BP is lacking. The pyrophosphate-dependent fructose 6-phosphate kinase can utilize pyrophosphate, which is produced in the UDP-glucose pyrophosphorylase reaction.
Fru2,6BP is synthesized from fructose 6-phosphate by a specific kinase (fructose 6-phosphate 2-kinase) and is degraded hydrolytically to fructose 6-phosphate by a specific phosphatase (fructose 2,6-bisphosphatase). The cellular concentration of the regulatory metabolite Fru2,6BP is adjusted by regulation of the relative rates of synthesis and degradation. Triose phos-phate and 3-phosphoglycerate inhibit the synthesis of Fru2,6BP, whereas fructose 6-phosphate and phosphate stimulate synthesis and decrease hydrolysis. Consequently the increase of triose phosphate concentra-tion results in a decrease in the level of Fru2,6BP and thus in an increased affinity of the cytosolic fructose 1,6-bisphosphatase towards its substrate fructose 1,6-bisphosphate. Moreover, due to the equilibrium catalyzed by cytosolic aldolase, an increase in the triose phosphate concentration results in an increase in the concentration of fructose 1,6-bisphosphate. The simul-taneous increase in substrate concentration and substrate affinity has the effect that only after a threshold level of triose phosphate is reached, does the rate of sucrose synthesis increase following rising concentrations of tri-ose phosphate (Fig. 9.17). In this way the rate of sucrose synthesis can be adjusted effectively to the supply of triose phosphate.
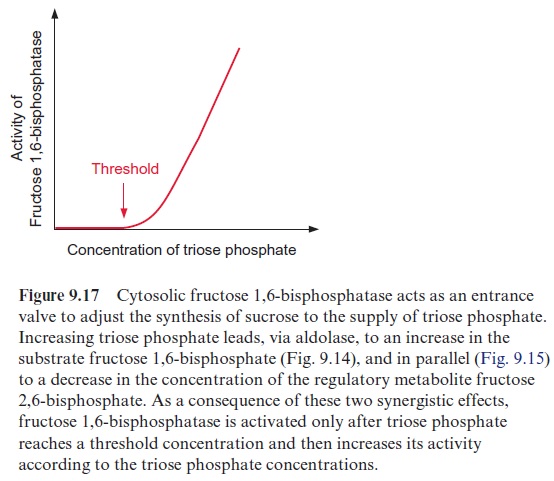
The principal mechanism of this regulation can be compared with an overflow valve. Only when a certain threshold concentration of triose phos-phate is overstepped can an appreciable metabolite flux via fructose 1,6-bisphosphatase occur. This mechanism ensures that the triose phosphate level in chloroplasts does not decrease below the minimum level which is required for the Calvin cycle reactions to proceed. When this threshold is reached, a further increase in triose phosphate results in a large increase in enzyme activity, whereby the surplus triose phosphate can be channeled very efficiently into sucrose synthesis.
Cytosolic fructose 1,6-bisphosphatase adjusts its activity, as shown above, not only to the substrate supply, but also to the demand for its product. With an increase in fructose 6-phosphate, the level of the regula-tory metabolite Fru2,6BP is increased by stimulation of fructose 6-phos-phate 2-kinase and simultaneous inhibition of fructose 2,6-bisphosphatase, resulting in a reduction of cytosolic fructose 1,6-bisphosphatase activity (Fig. 9.15).
Sucrose phosphate synthase is regulated by metabolites and by covalent modification
Sucrose phosphate synthase (Fig. 9.14) is also subject to strict metabolic control. This enzyme is activated by glucose 6-phosphate and is inhibited by phosphate. Due to hexose phosphate isomerase, the activator glucose 6-phosphate is in equilibrium with fructose 6-phosphate. In this equilib-rium the concentration of glucose 6-phosphate greatly exceeds the concen-tration of fructose 6-phosphate. Therefore, the change in the concentration of the substrate fructose 6-phosphate results in a much larger change in the concentration of the activator glucose 6-phosphate. In this way the activity of the enzyme is adjusted effectively to the supply of the substrate.
Moreover, the activity of sucrose phosphate synthase is altered by a covalent modification of the enzyme. At position 158 the enzyme has a serine residue, of which the OH-group is phosphorylated by a special pro-tein kinase, termed sucrose phosphate synthase kinase (SPS kinase) and is dephosphorylated by the corresponding SPS phosphatase (Fig. 9.18a). The SPS phosphatase is inhibited by okadaic acid, an inhibitor of protein phos-phatases of the so-called 2A type (not discussed in more detail here). The activity of SPS kinase is probably regulated by metabolites such as glucose 6-phosphate.
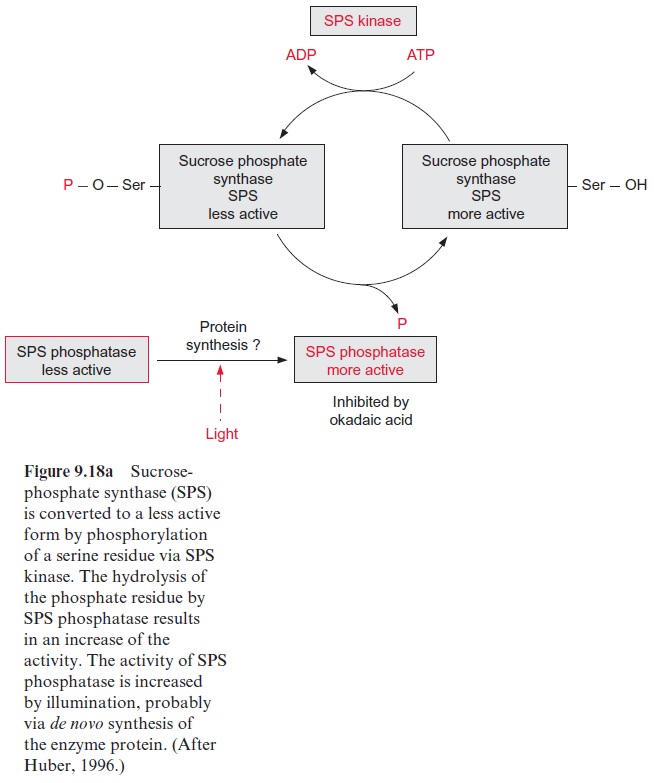
The phosphorylated form of sucrose phosphate synthase is less active than the dephosphorylated form. The activity of the enzyme is adjusted by the relative rates of its phosphorylation and dephosphorylation. When leaves are illuminated the activity of SPS phosphatase increases and thus the sucrose phosphate synthase is converted into the more active form. The mechanism for this is still not fully known. It is discussed that the decrease of SPS phosphatase activity during darkness is due to a lowered rate of the synthesis of the SPS phosphatase. In position 424 sucrose phosphate syn-thase has a second serine residue, which is phosphorylated by another pro-tein kinase (activated by osmotic stress), resulting in an activation of SPS. Thus the regulation of SPS is very complex. The phosphorylation of one serine residue by the corresponding protein kinase causes an inhibition, while the phosphorylation of another serine residue by a different pro-tein kinase leads to activation. Moreover, SPS has a third phosphorylation site, to which a 14.3.3 protein is bound (similarly as in the case of nitrate reductase). The physiological role of this binding remains to be resolved.
Partitioning of assimilates between sucrose and starch is due to the interplay of several regulatory mechanisms
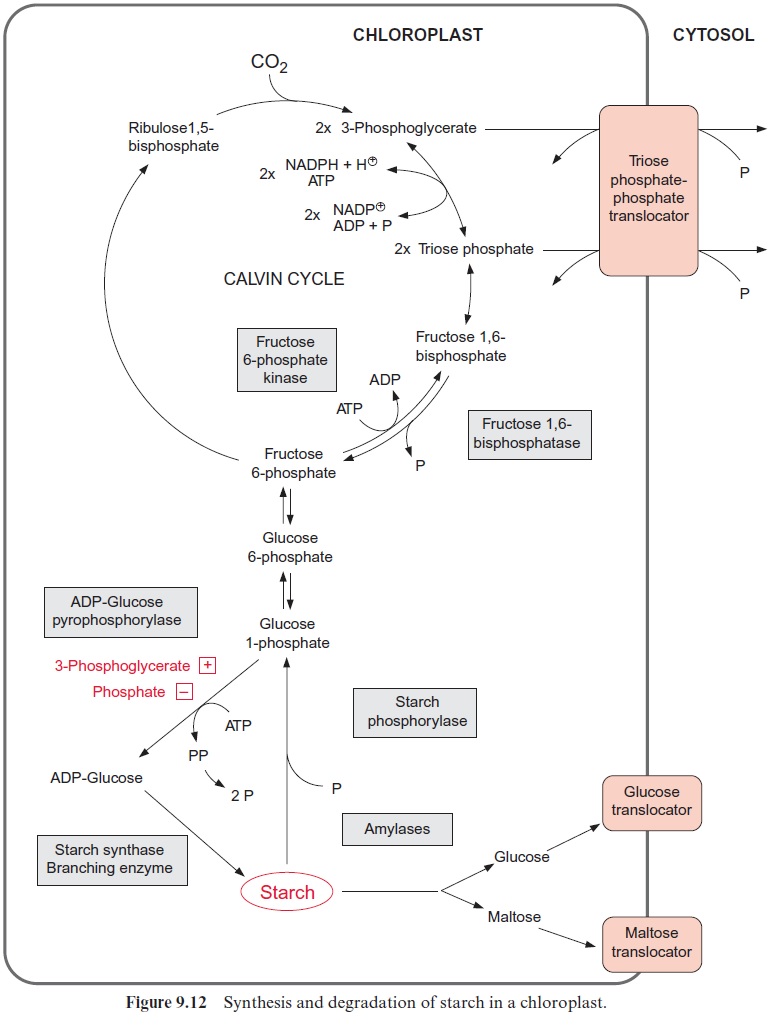
The preceding section discussed various regulatory processes involved in the regulation of sucrose synthesis. Metabolites acting as enzyme inhibi-tors or activators can adjust the rate of sucrose synthesis immediately to the prevailing metabolic conditions in the cell. Such an immediate response is called fine control. The covalent modification of enzymes, influenced by diurnal factors and probably also by phytohormones , results in a general regulation of metabolism according to the metabolic demand of the plant, including partitioning of assimilates between sucrose, starch, and amino acids . Thus, slowing down sucrose synthesis, which results in an increase in triose phosphate and also of 3-phosphoglycerate, can lead to an increase in the rate of starch synthesis (Fig. 9.12). During the day a large part of the photo assimilates is deposited temporarily in the chloroplasts of leaves as transitory starch, to be converted during the fol-lowing night to sucrose and delivered to other parts of the plant. However, in some plants, such as barley, during the day large quantities of the photo assimilates are stored as sucrose in the leaves. Therefore during darkness the rate of sucrose synthesis varies in leaves of different plants.
Trehalose is an important signal mediator
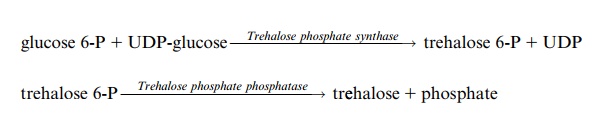
For a long time trehalose (Fig. 9.18b), occurring in plant cells only in small concentrations, was regarded as being of minor importance. Only recently it was shown that trehalose and trehalose phosphate are very important signal metabolites involved in the regulation of plant metabolism. Precursors for trehalose synthesis are glucose 6-phosphate and UDP-glucose:
The importance of trehalose is illustrated by the fact that the Arabidopsis genome provides more genes for its synthesis than for the synthesis of sucrose. Trehalose and trehalose phosphate stimulate in plants the synthe-sis of starch, increase the resistance to dryness, and are involved in trigger-ing flowering and maturation of embryos. The mechanisms of these actions remain to be elucidated.
Related Topics