Chapter: Plant Biochemistry: Nitrate assimilation is essential for the synthesis of organic matter
The reduction of nitrate to NH3 proceeds in two reactions
The reduction of nitrate to NH3 proceeds in two reactions
Nitrate is assimilated in the leaves and also in the roots. In most fully grown herbaceous plants, nitrate assimilation occurs primarily in the leaves, although nitrate assimilation in the roots often plays a major role at an early growth state of these plants. In contrast, many woody plants (e.g., trees, shrubs), as well as legumes such as soybean, assimilate nitrate mainly in the roots.
The transport of nitrate into the root cells proceeds via symport with two protons (Fig. 10.1). A proton gradient across the plasma membrane, generated by an H+ -P-ATPase , drives the uptake of nitrate against a concentration gradient. The ATP required for the formation of the proton gradient is provided mostly by mitochondrial respiration. When inhibitors or uncouplers of respiration abolish mitochondrial ATP synthe-sis in the roots, nitrate uptake normally comes to a stop. Root cells contain several nitrate transporters in their plasma membrane; among these are a transporter with a relatively low affinity (half saturation >500×10–6 mol/L nitrate) and a transporter with a very high affinity (half saturation 20–100×10–6 mol/L nitrate), where the latter is induced only when required by metabolism. In this way the capacity of nitrate uptake into the roots is adjusted to the environmental conditions. The efficiency of the nitrate uptake systems enables plants to grow when the external nitrate concentration is as low as 10×10–6 mol/L.
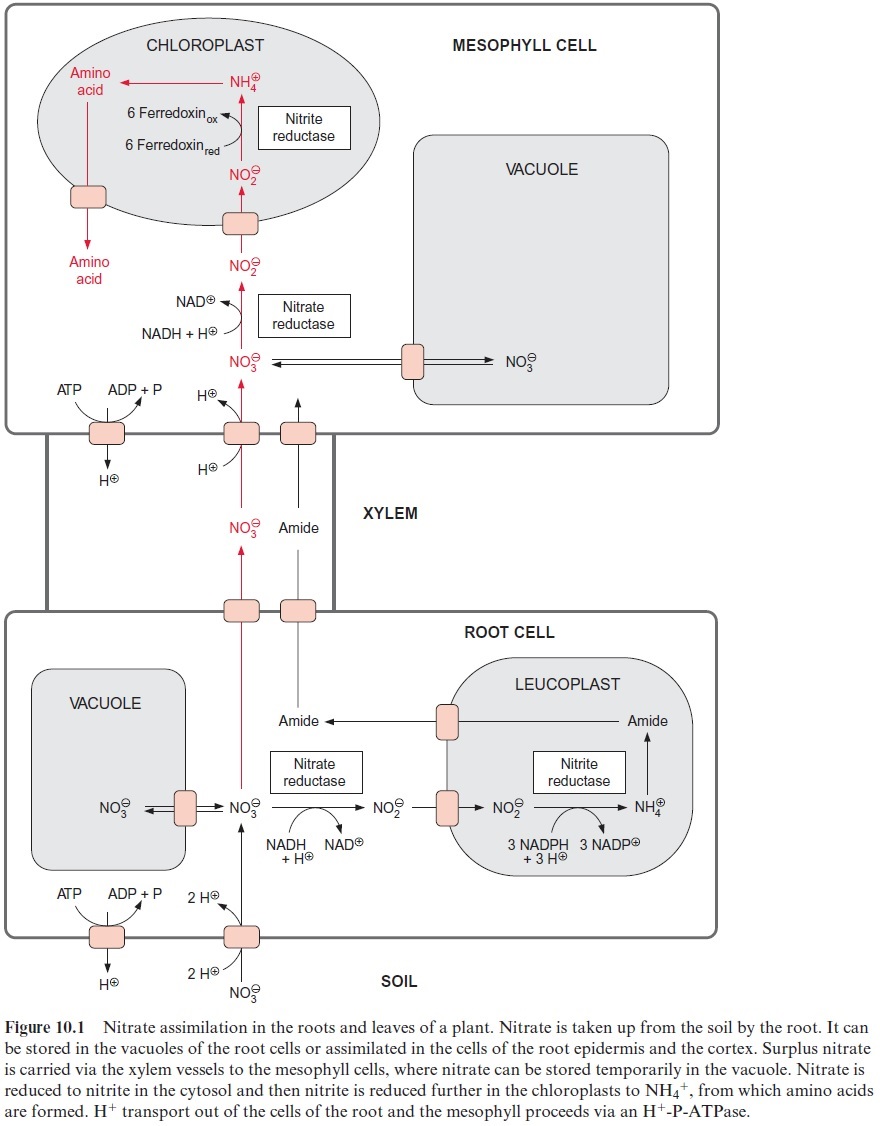
The nitrate taken up into the root cells can be stored temporarily in the vacuole. Nitrate is reduced to NH4+ in the epidermal and cortical cells of the root. This NH4+ is used mainly for the synthesis of glutamine and asparagine (collectively named amide in Fig. 10.1). These two amino acids can be transported to the leaves via the xylem vessels. However, when the capacity for nitrate assimilation in the roots reaches its maximum, nitrate is released from the roots into the xylem vessels and is carried by the transpiration stream to the leaves. The uptake into the mesophyll cells occurs probably also by a proton symport. Large quantities of nitrate can be stored in the vacuole. This vacuolar store may be emptied by nitrate assimilation during the day and replenished during the night. Thus for instance in spinach leaves the highest nitrate content is found in the early morning.
The nitrate in the mesophyll cells is first reduced to nitrite by nitrate reductase present in the cytosol and then to NH4+ by nitrite reductase in the chloroplasts (Fig. 10.1).
Nitrate is reduced to nitrite in the cytosol
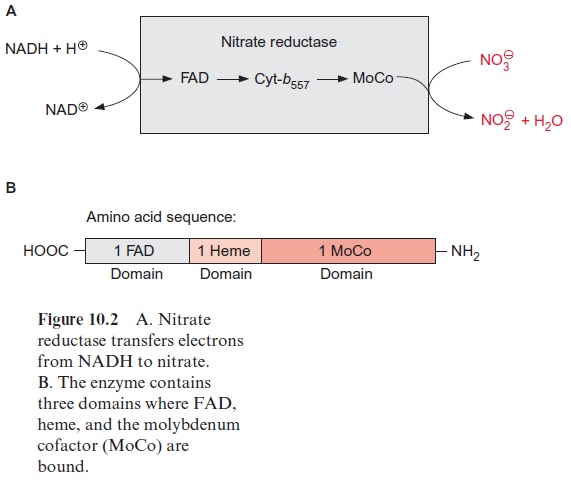
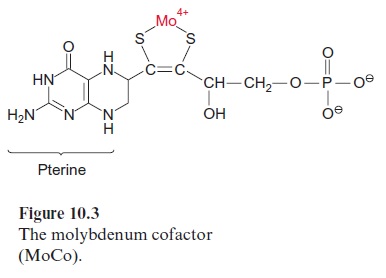
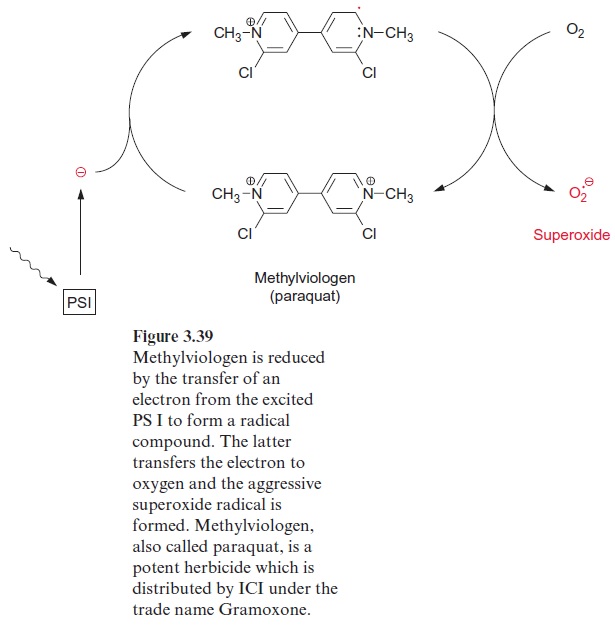
Nitrate reduction uses mostly NADH as reductant, although some plants contain a nitrate reductase reacting with NADPH as well as with NADH. The nitrate reductase of higher plants consists of two identical subunits. The molecular mass of each subunit varies from 99 to 104 kDa, depending on the species. Each subunit comprises an electron transport chain (Fig. 10.2) consisting of one flavin adenine dinucleotide molecule (FAD), one heme of the cytochrome-b type (cyt-b557), and one cofactor containing molybdenum (Fig. 10.3). The latter is a pterin with a side chain to which the molybde-num is attached by two sulfur bonds and is called the molybdenum cofactor, abbreviated MoCo. The bound Mo atom probably changes between oxida-tion states +IV and +VI. The three redox carriers of nitrate reductase are each covalently bound to the subunit of the enzyme. The protein chain of the subunit can be cleaved by limited proteolysis into three domains, each of which contains only one of the redox carriers. These separated domains, as well as the holoenzyme, are able to catalyze via their redox carriers elec-tron transport to artificial electron acceptors (e.g., from NADPH to Fe+++ ions via the FAD domain or from reduced methylviologen (Fig. 3.39) to nitrate via the Mo domain). Moreover, nitrate reductase reduces chlorate (ClO3- ) to chlorite (ClO2–). The latter is a very strong oxidant and there-fore highly toxic to plant cells. In the past chlorate was used as an inexpen-sive nonselective herbicide for keeping railway tracks free of vegetation.
The reduction of nitrite to ammonia proceeds in the plastids
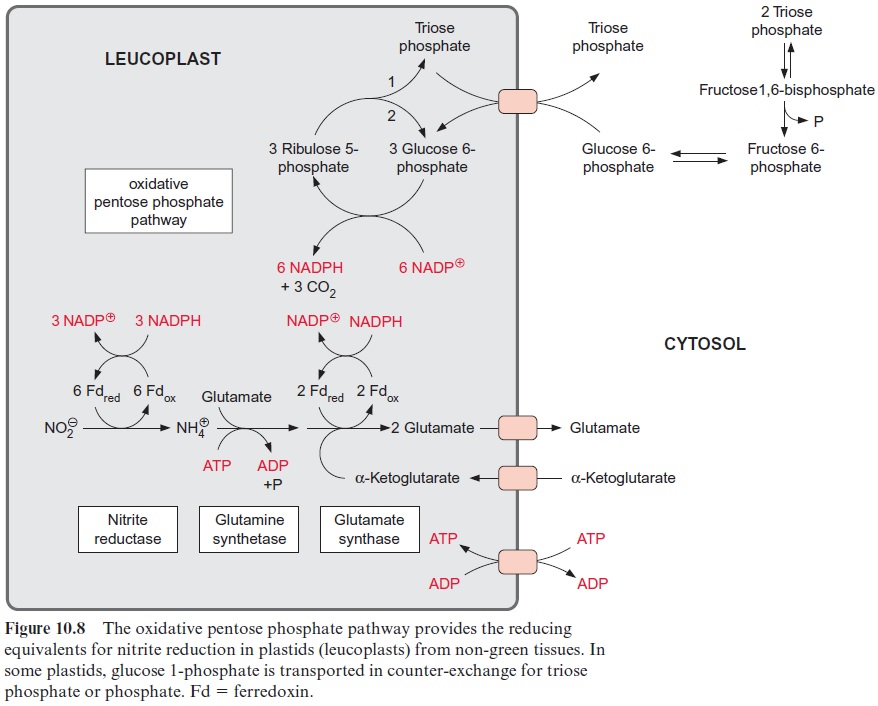
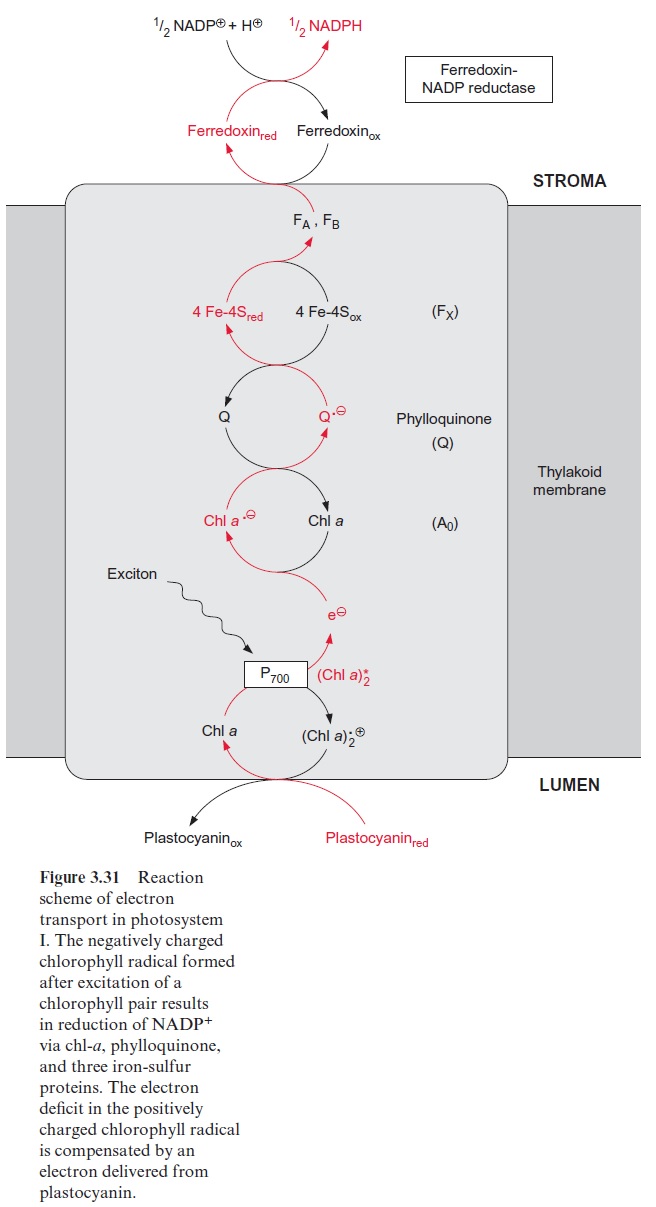
The reduction of nitrite to ammonia requires the uptake of six elec-trons. This reaction is catalyzed by only one enzyme, the nitrite reductase (Fig. 10.4), which is located exclusively in plastids. This enzyme utilizes reduced ferredoxin as electron donor, which is supplied as a product of photosynthetic electron transport by photosystem I (Fig. 3.31). To a much lesser extent, the reduced ferredoxin can also be provided during darkness via reduction by NADPH. The latter is generated by the oxida-tive pentose phosphate pathway present in chloroplasts and leucoplasts (Figs. 6.21, 10.8).
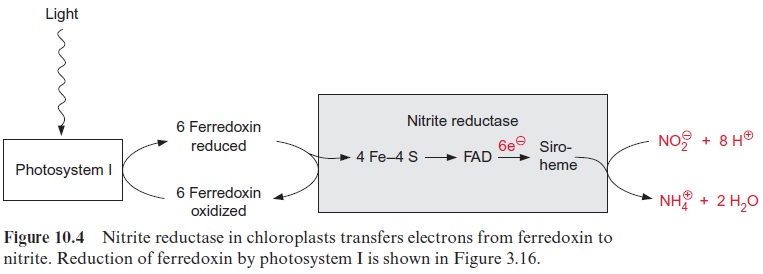
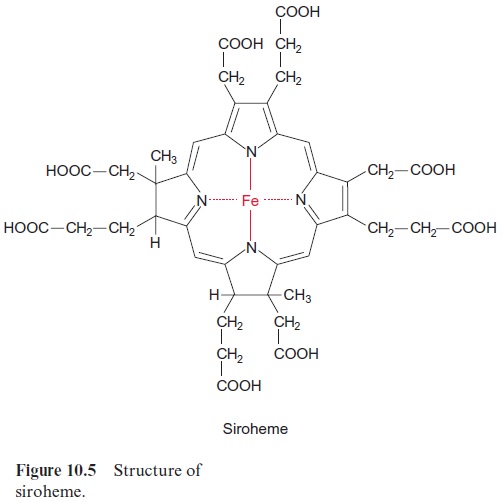
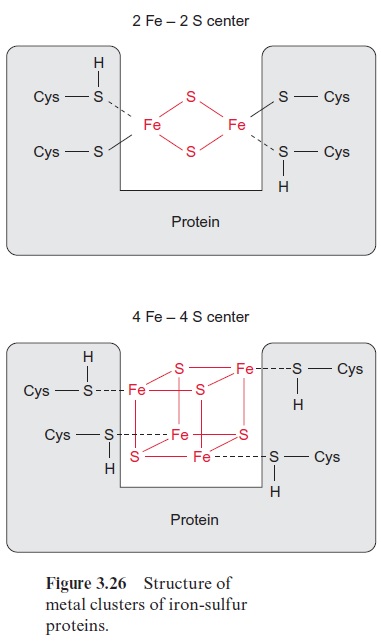
Nitrite reductase contains a covalently bound 4Fe-4S cluster (see Fig. 3.26), one molecule of FAD, and one siroheme. Siroheme (Fig. 10.5) is a cyclic tetrapyrrole with one Fe atom in the center. Its structure is different from that of heme as it contains additional acetyl and propionyl residues deriving from pyrrole synthesis.
The 4Fe-4S cluster, FAD, and siroheme form an electron transport chain by which electrons are transferred from ferredoxin to nitrite. Nitrite reductase has a very high affinity for nitrite. The capacity for nitrite reduc-tion in the chloroplasts is much greater than that for nitrate reduction in the cytosol. Therefore all nitrite formed by nitrate reductase can be com-pletely converted to ammonia. This is important since nitrite is toxic to the cell. It forms diazo compounds with amino groups of nucleobases (R–NH2), which are converted into alcohols with the release of nitrogen.
R - NH 2 + NO 2- → [R - N = N – OH + OH- ] → R – OH + N 2 + OH-
Thus, for instance, cytosine can be converted to uracil. This reaction can lead to mutations in nucleic acids. The very efficient reduction of nitrite by plastid nitrite reductase prevents nitrite from accumulating in the cell.
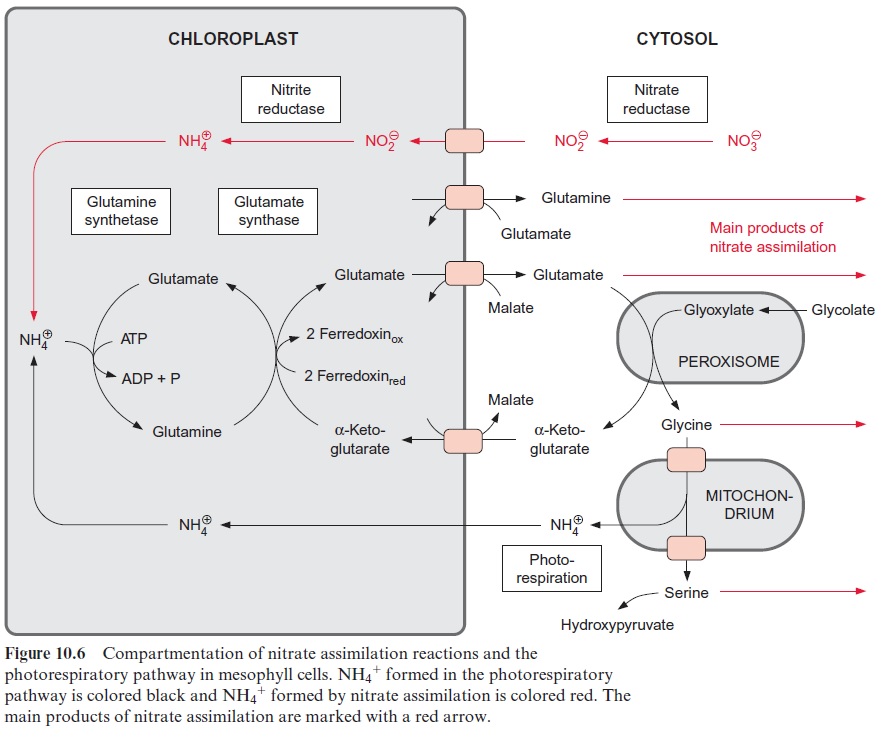
The fixation of NH4+ proceeds in the same way as in the photorespiratory cycle
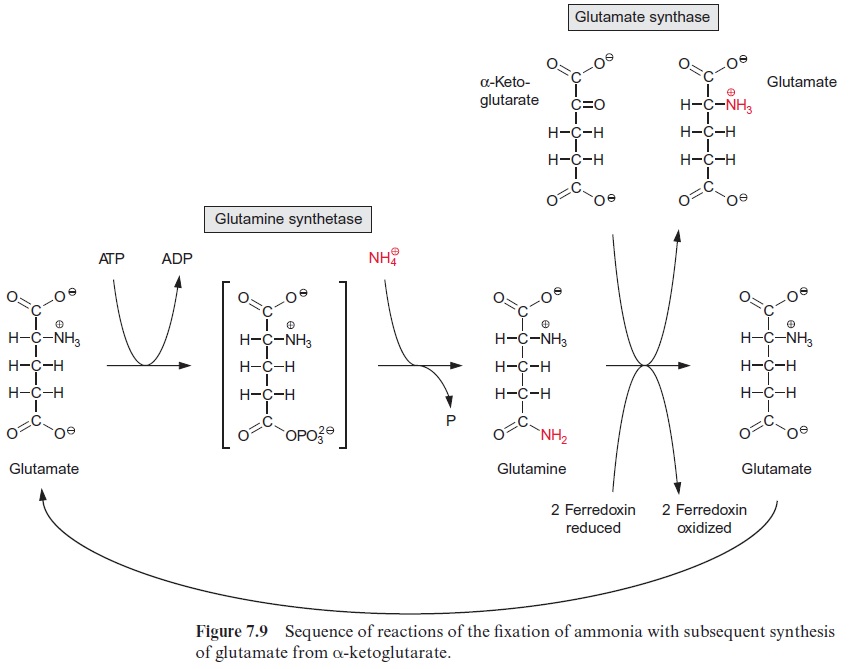
Glutamine synthetase in the chloroplasts transfers the newly formed NH4+ at the expense of ATP to glutamate, forming glutamine (Fig. 10.6). The activ-ity of glutamine synthetase and its affinity for NH4+ (Km≈5 · 10–6 mol/L) are so high that the NH4+ produced by nitrite reductase is completely assimilated into glutamine. Glutamine synthetase also fixes the NH4+ released during photorespiration (see Fig. 7.9). Due to the high rate of pho-torespiration, the amount of NH4+ produced by the oxidation of glycine is about 5 to 10 times higher than generated by nitrate assimilation. Thus only a minor proportion of glutamine synthesis in the leaves results from nitrate assimilation. Leaves also contain an isoenzyme of glutamine synthe tase in their cytosol.
Glufosinate (Fig. 10.7), a substrate analogue of glutamate, is a strong inhibitor of the glutamine synthetase. When glufosinate is applied to plants the synthesis of glutamine is inhibited and subsequently toxic levels of ammonia accumulate. Glufosinate is a herbicide and com-mercially available under the trade name Basta (Bayer Crop Science). This herbicide degrades rapidly in the soil, without the accumulation of toxic degradation products. Recently glufosinate-resistant crop plants have been generated by genetic engineering, enabling the use of glufosinate as a selec-tive herbicide for weed control in growing cultures .
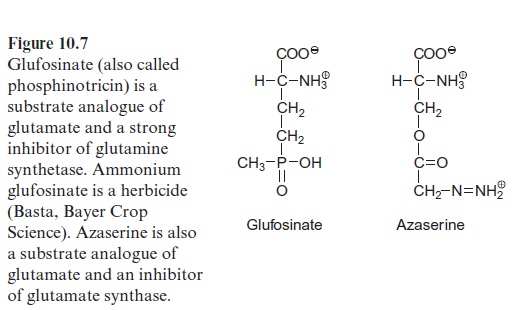
Glutamine together with α-ketoglutarate is converted by glutamate syn-thase (also called glutamine-oxoglutarate aminotransferase, abbreviated GOGAT), to two molecules of glutamate (see also Fig. 7.9). Ferredoxin is used as reductant in this reaction. Some chloroplasts and leucoplasts also contain an NADPH-dependent glutamate synthase. Glutamate synthases are inhibited by the substrate analogue azaserine (Fig. 10.7), which is toxic to plants.
α-Ketoglutarate, which is required for the glutamate synthase reaction, is transported into the chloroplasts by a specific translocator in counter-exchange for malate, and the glutamate formed is transported out of the chloroplasts into the cytosol by another translocator, also in exchange for malate (Fig. 10.6). Yet another translocator in the chloroplast enve-lope transports glutamine in counter-exchange for glutamate, enabling the export of glutamine from the chloroplasts.
Related Topics