Chapter: Plant Biochemistry: Nitrate assimilation is essential for the synthesis of organic matter
Glutamate is the precursor for chlorophylls and cytochromes
Glutamate is the precursor for chlorophylls and cytochromes
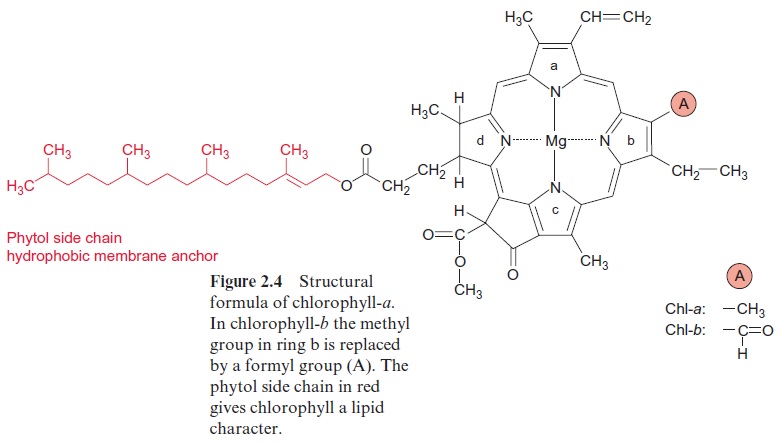
Chlorophyll amounts to 1% to 2% of the dry matter of leaves. Its synthe-sis proceeds in the plastids. As shown in Figure 2.4, chlorophyll consists of a tetrapyrrole ring with magnesium as the central atom and with a phytol side chain as a hydrophobic membrane anchor. Heme, likewise a tetrapyr-role, but with iron as the central atom, is a constituent of cytochromes and catalase.
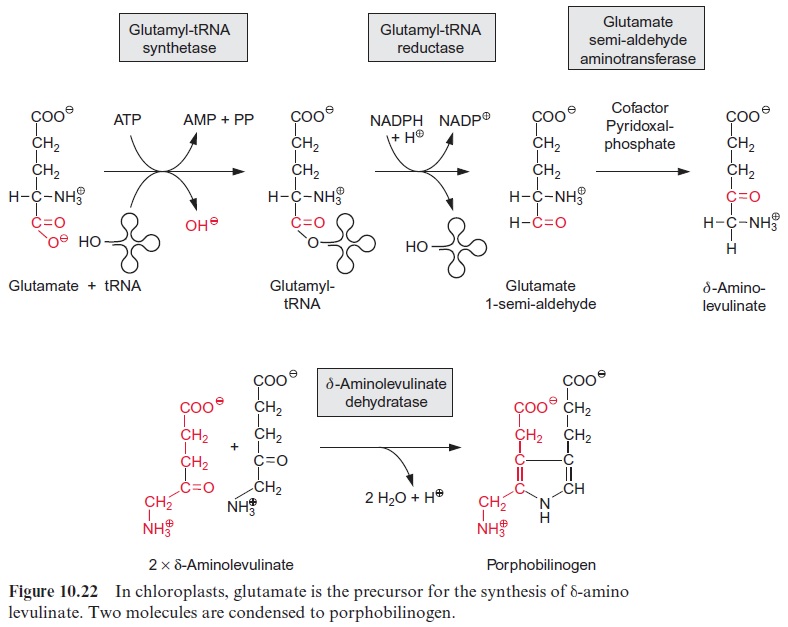
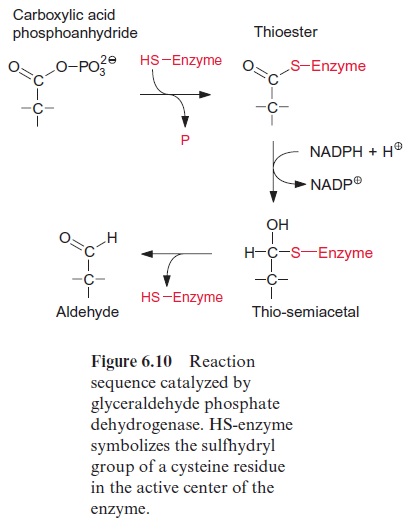
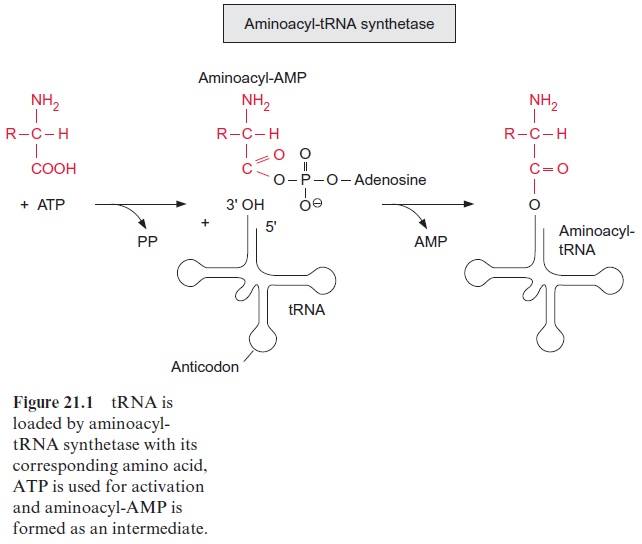
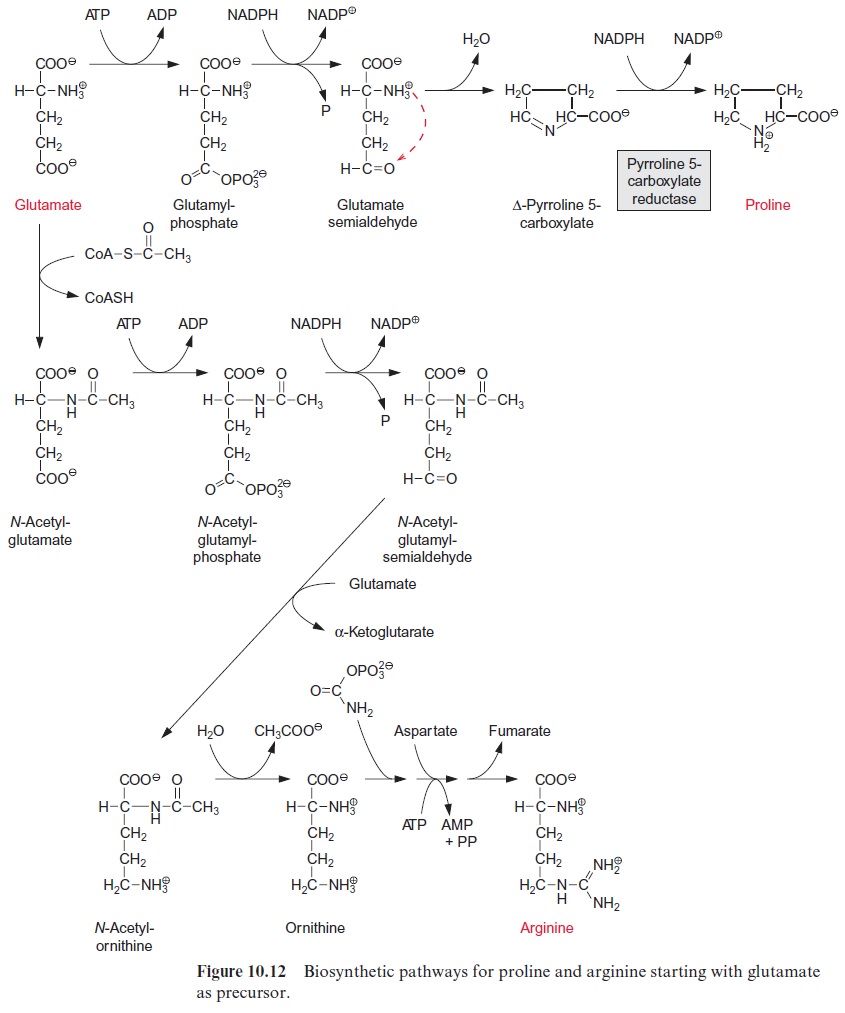
Porphobilinogen, a precursor for the synthesis of tetrapyrroles, is formed by the condensation of two molecules of δ-amino levulinate. -Amino lev-ulinate is synthesized in animals, yeast, and some bacteria from succinyl-CoA and glycine, accompanied by the liberation of CoASH and CO2. In contrast, the synthesis of -amino levulinate in plastids, cyanobacteria, and many eubacteria proceeds by reduction of glutamate. As discussed in sec-tion 6.3, the difference in redox potentials between a carboxylate and an aldehyde is so high that a reduction of a carboxyl group by NADPH is only possible when this carboxyl group has been previously activated (e.g., as a thioester (Fig. 6.10) or as a mixed phosphoric acid anhydride (Fig. 10.12)). In the plastid -amino levulinate synthesis, glutamate is activated in a very unusual way by a covalent linkage to a transfer RNA (tRNA) (Fig. 10.22). This tRNA for glutamate is encoded in the plastid genome and is involved in the plastids in the synthesis of δ-amino levulinate as well as in protein biosynthesis. As in protein biosynthesis (see Fig. 21.1), the linkage of the carboxyl group of glutamate to tRNA is accompanied by consumption of ATP. During reduction of glutamate tRNA by glutamate tRNA reductase, tRNA is liberated and in this way the reaction becomes irreversible. The glutamate 1-semi-aldehydethus formed is converted to δ-amino levulinate by an aminotransferase with pyridoxal phosphate as a prosthetic group. This reaction proceeds according to the same mechanism as the ami-notransferase reaction shown in Figure 7.4, the only difference being that the amino group (as amino donor) and the keto group (as amino acceptor) is present in the same molecule.
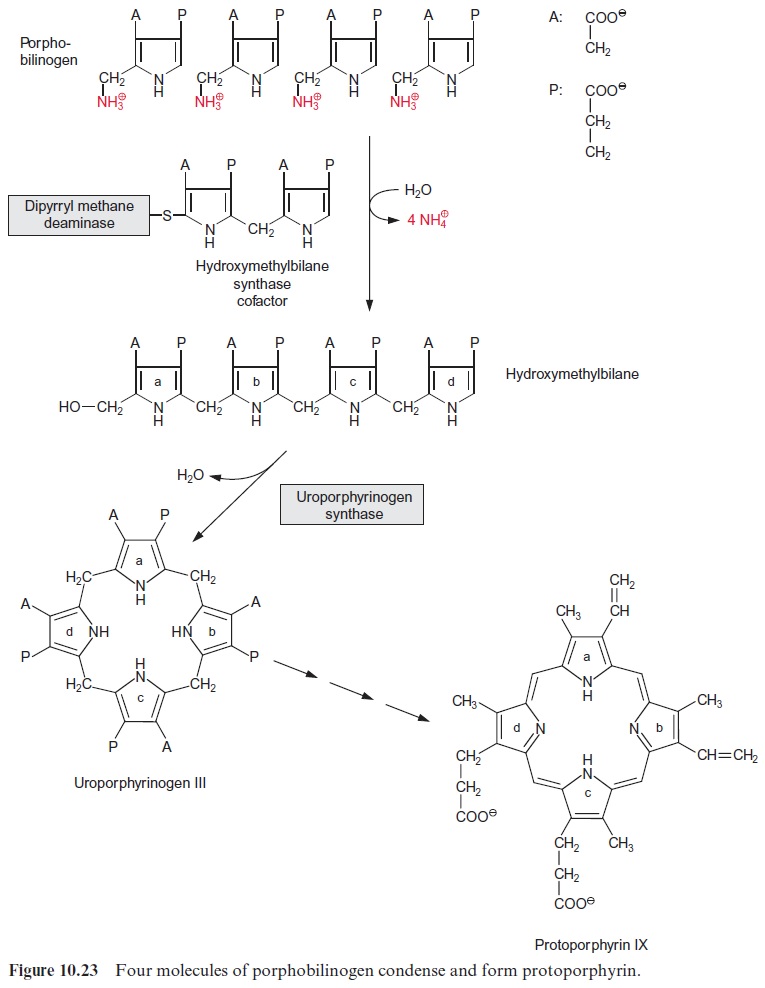
Two molecules of δ-amino levulinate condense to form porphobilino-gen (Fig. 10.22). The open-chain tetrapyrrole hydroxymethylbilan is syn-thesized from four molecules of porphobilinogen via hydroxymethylbilan synthase (Fig. 10.23). The enzyme contains a dipyrrole as cofactor. After the exchange of the two side chains on ring d the closure of the tetrapyrrole ring produces uroporphyrinogen III. Subsequently, protoporphyrin IX is formed by reaction with a decarboxylase and two oxidases (not shown in detail). Mg++ is incorporated into the tetrapyrrole ring by magnesium che-latase and the resultant Mg-protoporphyrin IX is converted by three moreenzymes to protochlorophyllide. The tetrapyrrole ring of protochlorophyl-lide contains the same number of double bonds as protoporphyrin IX. The reduction of one double bond in ring d by NADPH yields chlorophyllide.
Protochlorophyllide oxido-reductase, which catalyzes this reaction, is only active when protochlorophyllide is activated by absorption of light. The transfer of a pyrophosphate activated phytyl chain to protochlorophyllide via a prenyl transferase (chlorophyll synthetase) completes the synthesis of chlorophyll.
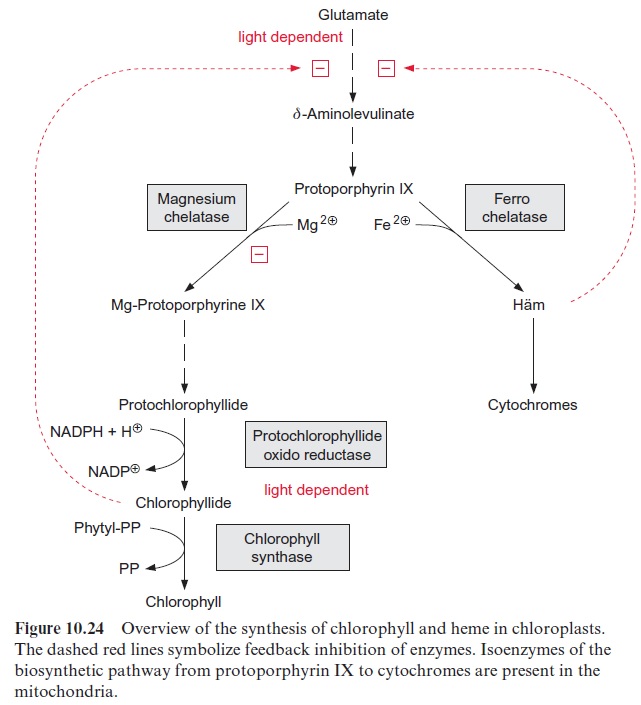
The light dependence of the protochlorophyllide reductase allows a developing shoot to green only when it reaches the light. Also, the synthe-sis of the chlorophyll binding proteins of the light harvesting complexes is light-dependent. The exceptions are some gymnosperms (e.g., pine), in which protochlorophyllide reduction as well as the synthesis of chloro-phyll binding proteins also progresses during darkness. Unprotected and unbound porphyrins may lead to photochemical cell damage. It is therefore important that intermediates of chlorophyll biosynthesis do not accumu-late. To prevent this, the synthesis of δ-amino levulinate is light-dependent, but the mechanism of this regulation is not yet fully understood. Moreover, δ-amino levulinate synthesis is subject to feedback inhibition by chloro-phyllide. The end products protochlorophyllide and chlorophyllide inhibit magnesium chelatase (Fig. 10.24). Moreover, intermediates of chlorophyll synthesis control the synthesis of light harvesting proteins via the regulation of gene expression.
Protophorphyrin is also precursor for heme synthesis
Incorporation of an iron ion into protoporphyrin IX by a ferro-chelatase results in the formation of heme. By assembling the heme with apopro-teins, chloroplasts are able to synthesize their own cytochromes and phy-tochromes. Also, mitochondria possess the enzymes for the biosynthesis of cytochromes from protoporphyrin IX, but the corresponding enzyme proteins are different from those in the chloroplasts. Present knowledge suggests that the heme for mitochondrial cytochromes is synthesized in the mitochondria whereas the heme synthesis of chloroplasts not only serves their own demand but also provides hemes for cytosolic heme proteins.
Related Topics